Novel approach is improving presurgical evaluation
By Z. Irene Wang, PhD, and Stephen E. Jones, MD, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The widespread use of MRI has significantly impacted the diagnosis and surgical treatment of epilepsy, where identification of the underlying epileptogenic pathology plays a key role. Even so, a large percentage of patients who undergo evaluation for epilepsy surgery still have a conventional MRI showing no abnormality. These “MRI-negative” or “nonlesional” patients, approximately 40% of the total epilepsy surgery population, typically require expensive and invasive intracranial electroencephalography (ICEEG) to identify the epileptogenic zone. Further improvement of the sensitivity of MRI to subtle epileptogenic pathologies would therefore be of great benefit. With the advent of continuously increasing computing power and memory, the interpretation of MRI data can now be markedly improved by computer-assisted methods designed to extract information not easily seen by visual analysis.
In Cleveland Clinic’s Epilepsy Center, the imaging research team specializes in using computer-assisted MRI post-processing to improve noninvasive epilepsy localization. This new technology has positively impacted many patients with epilepsy, particularly those whose MRIs were negative by conventional visual analysis. Recently, the research team has further enhanced MRI post-processing techniques with artificial intelligence and machine learning to build robust workflows for automated detection of epileptogenic lesions.
Several years ago, the Epilepsy Center imaging research team published a study indicating that the main pathology in nonlesional epilepsy is focal cortical dysplasia (FCD).1 FCD lesions are usually characterized by subtle MRI features and can be very difficult to see on 3T clinical MRI scans. In another study published by our imaging research team, involving a cohort of 150 MRI-negative surgical patients,2 voxel-based MRI post-processing revealed positive findings in 41% of patients, and complete resection of these positive regions correlated positively with seizure-free outcome. A few additional studies published by our team further demonstrated the usefulness of voxel-based MRI post-processing in cohorts with very challenging epilepsies arising from the orbitofrontal cortex,3 the cingulate cortex4 and the operculoinsular regions.5 Subtle lesions in these areas are difficult to identify visually due to the regions’ complex cortical convolutions. In addition, because of these regions’ deep locations far from the brain surface, commonly used localization tools such as scalp EEG may be unhelpful or even misleading.
Advertisement
Our studies have shown that MRI post-processing can add another source of essential data to the evaluation of these very challenging epilepsies. In addition, previous studies by our team also showed benefits of computer-assisted MRI post-processing in the pediatric population6 and in patients with recurrent seizures after previous unsuccessful epilepsy surgery.7 Our published studies provided validation of the MRI post-processing methods, enabling us to integrate them into the routine presurgical evaluation of patients with MRI-negative medically intractable epilepsies in the Epilepsy Center. With minimal extra cost and no additional tests or risks to the patient, this process has shed light on many difficult cases.
Recent advanced developments in artificial intelligence and machine learning are revolutionizing the way large volumes of patient data are interpreted. Equipped with large surgical volumes, MRI data and pathological confirmation of resected tissue, the Epilepsy Center imaging research team is devoting substantial efforts to using machine learning algorithms to inform the way epileptic lesions are detected and delineated.
Figure 1 shows results from voxel-based morphometric post-processing based on 3T T1-weighted MRI from a patient with histologically confirmed FCD type IIb. Z-score maps of three cortical features were fed into a neural network classifier that was trained on 90 patients with histologically confirmed FCD and 350 controls. The classifier produced a probability map that shows successful automated detection of a subtle lesion in the insular cortex; the lesion was so small and subtle that it was missed by visual analysis many times.
Advertisement
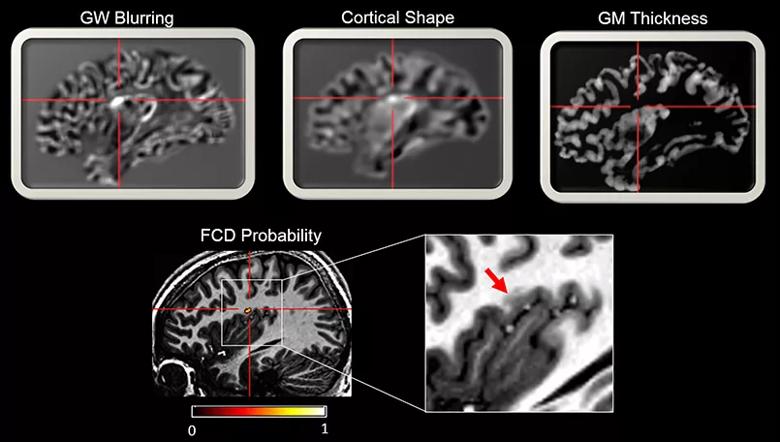
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3d5c193d-3f90-4f18-9bc0-7915dd668eb5/Figure-1_jpg)
Figure 1. Example images showing multiple cortical features generated by voxel-based morphometric MRI post-processing based on 3T T1-weighted MRI from a patient with histologically confirmed FCD type IIb. Z-score maps of the three cortical features were fed into a neural network classifier trained on 90 patients with histologically confirmed FCD and 350 healthy controls. The classifier produced a probability map that shows successful automated detection of the lesion. GW = gray-white; GM = gray matter; FCD = focal cortical dysplasia.
Figure 2 shows an automated multivariate surface-based morphometry analysis methodology combined with machine learning for automated FCD detection. Compared with the voxel-based approach shown in Figure 1, surface-based morphometry analysis was capable of generating a larger number of feature maps, such as cortical thickness, gray-white matter blurring, sulcal depth, curvature and cortical shape deformation. Additionally, asymmetry maps were generated to account for inherent left-right differences in each individual brain. These features were used as inputs to a machine learning algorithm realized by a nonlinear neural network classifier for automated lesion detection, yielding output of clusters with a high likelihood of FCD abnormality. The current method already showed robust performance on 3T MRI data, as demonstrated by a multicenter study led by the Epilepsy Center imaging research team.8
Specifically, Figure 2 presents findings from a patient whose subtle MRI lesion was successfully and automatically recognized by the current method. The detected lesion was confirmed by expert radiologist review and fit with the patient’s multimodal evaluation data (PET, ictal SPECT and ICEEG). Notably, multifocal appearance of individual features did not preclude the machine learning algorithm from successfully detecting one single lesion, as exemplified in Figure 2.
Advertisement
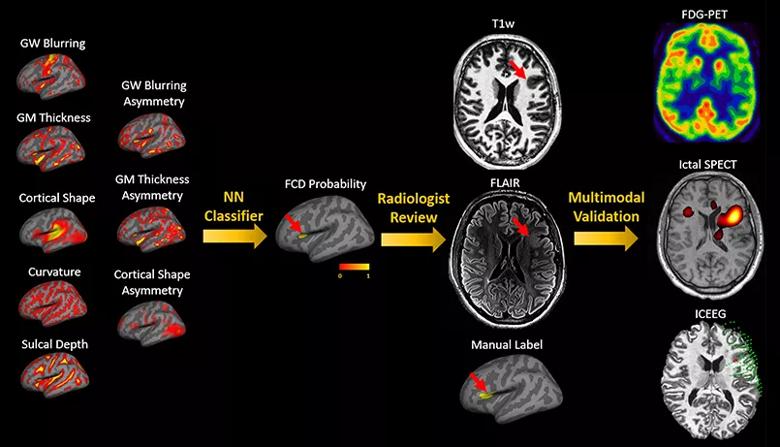
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dbe84433-f6ae-41ea-bfcb-6ec7133c1907/Figure-2_jpg)
Figure 2. Images from an example patient showing the effectiveness of 3T surface-based morphometry for FCD detection. On the left are feature maps constructed from 3T T1-weighted (T1w) MRI data, which are used as the input to machine learning using a neural network (NN) classifier. The training set was 60 patients with histopathologically confirmed FCD and 120 healthy controls. The NN classifier output final cluster is shown on inflated brain surface due to deep lesion location. Detailed review by an expert neuroradiologist confirmed the presence of the lesion detected by machine learning; the lesion was located in the left anterior insular region (red arrows) with thickened cortex and abnormally deep sulcus on both T1w and FLAIR sequences. The success of detection was further validated by overlap between other presurgical evaluation data, i.e., FDG-PET hypometabolism, hyperperfusion indicated by ictal SPECT and ictal onset from ICEEG evaluation (ICEEG ictal onset is shown by red spheres; implanted grids and depth electrodes are shown by green spheres).
Using this surface-based morphometry and machine learning approach, 61 patients were evaluated who had pharmacoresistant epilepsy and histologically proven FCD type II.8 The patients had been evaluated at three different epilepsy centers using three different MRI machines. The neural network classifier achieved a sensitivity of 74% and a specificity of 90%. Subgroup analysis showed similarly high sensitivity among patients included from the different epilepsy centers, and there was no statistically significant difference between children and adults. Overall, our findings provide evidence that the fully automated surface-based morphometry and machine learning approach could offer substantial gains in FCD detection in the presurgical evaluation of patients with pharmacoresistant epilepsy.
Advertisement
The machine learning approach allows for “fusion” of many MRI features, and it is often necessary to understand which features were the main driving force for correct classification. As shown in Figure 3, our data suggest that correct classification was largely based on gray-white matter blurring, cortical shape and cortical thickness, which showed the greatest group-level difference relative to normal control cortex. In the future, this methodology can be expanded to include additional novel imaging features as they become available, thereby continuing to increase the accuracy of subtle lesion identification.
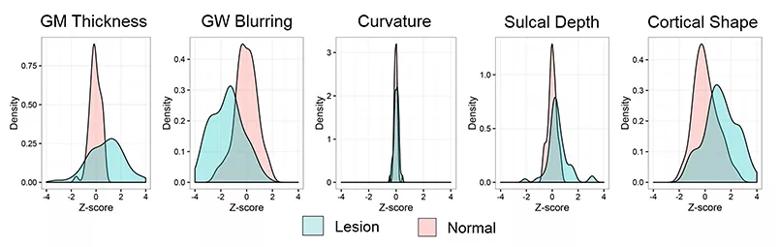
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/15c1b1c7-73e1-412c-9da7-f7ba4fbe7538/Figure-3_jpg)
Figure 3. Quantitative evaluation of features in lesions as compared with normal cortex group. Kernel density plots show the distribution of mean feature values in lesional and homotopic normal cortices across the cohort of FCD patients. Green indicates lesion profile; pink indicates contralesional profile (homotopic normal cortex). The homotopic cortex profile was calculated from 120 normal controls.
As expected, our study showed that the sensitivity and specificity of the integrated surface-based MRI morphometry and machine learning approach were heavily influenced by the size of the training group of patients and normal controls. The higher the number of patients included in the training group, the higher the sensitivity and specificity tended to be. This motivated our research team to retrospectively review MRI and histopathology data from our large surgical volume for labeling of the training set. The need for large amounts of training data also necessitates joining forces with international consortia so that labeled data from multiple centers may be available for testing and validation.
Machine learning models (soon with deep neural networks, which are now the state-of-the-art machine learning models across a variety of areas [i.e., “deep learning”]) have the inherent advantage of training on multimodal datasets and being able to handle partial overlap between the different modalities. The opportunity is well suited to epilepsy, due to the large volumes of multimodal data generated during presurgical evaluation, including data from MRI, CT, PET, SPECT, MEG, scalp EEG and ICEEG, as well as clinical information.
Features within the MRI data that are not obvious to the human eye may emerge from the machine/deep learning models as highly determinative of lesion location. Solid validation of these models will come from clinical phenotyping data before and after epilepsy surgery, which will make the machine/deep learning models well grounded. Eventually, machine/deep learning techniques will lead to the construction of a well-trained computer algorithm that is based on retrospectively validated data derived from a very large number of patients. Such an algorithm could help inform clinical recommendations on an individual basis. This strategy will have lasting impact across the country and beyond by markedly improving epilepsy seizure outcomes and increasing the number of patients who can be deemed favorable candidates for potentially curative surgery.
Dr. Wang is a staff scientist in Cleveland Clinic’s Epilepsy Center and joint staff in the Department of Biomedical Engineering, Cleveland Clinic Lerner Research Institute.
Dr. Jones is a staff neuroradiologist and Vice Chairman for Research and Academic Affairs in Cleveland Clinic’s Imaging Institute.
Advertisement
Ethical guidance provides guardrails so medical advances benefit patients
OCEANIC-STROKE results represent long-sought advance in secondary stroke prevention
Two studies from Cleveland Clinic may help advance the technology toward broader clinical use
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
An argument for clarifying the nomenclature
An expert talks through the benefits, limits and unresolved questions of an evolving technology
Recommendations on identifying and managing neurodevelopmental and related challenges
Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies