
Oncologic and functional outcomes are promising, but selection is key

Minimally invasive pancreas-kidney replacement reduces patient’s pain, expedites recovery

Cleveland Clinic is first to use the device, known formerly as the UroMonitor

First single-port renal vein transposition reduces recovery time and improves outcomes
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy

Approach offers a ‘middle ground’ between radical prostatectomy and active surveillance

American Urological Association presentation outlines the latest evolution of treatment protocols
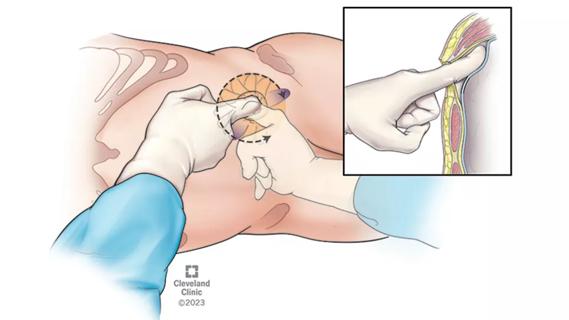
The low anterior access approach using the single-port robot is gaining attention within the field

Findings confirm microbial variability, offer new potential treatment strategy

AI histologic classifier reliably predicts clinical risk in men post-prostatectomy

Historic collaboration connects two Cleveland Clinic locations, enables real-time sharing of metrics and surgical progress
Advertisement
Advertisement