Cleveland Clinic pulmonologists address the role of measuring exhaled nitric oxide in the diagnosis and management of asthma and provide guidance for its appropriate use

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2cce6e26-e4fd-4051-9519-7218e72b5304/23-PUL-4172458-Hero_jpg)
Asthma
Note: This article is reprinted from the Cleveland Clinic Journal of Medicine (2023;90[6]:363-370).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Written by Payal Sen, MD, Sumita B. Khatri, MD and Vickram Tejwani, MD
Asthma is a heterogeneous pulmonary disease characterized by inflammation of the lower airways. In recent years, specific endotyping through biomarkers of airway inflammation has helped in guiding evaluation and assessment of the severity of asthma and in deciding on treatment.
One of the biomarkers is exhaled nitric oxide, which can now be measured in parts per billion (ppb). In this review, we discuss current care guidelines regarding the role of measuring exhaled nitric oxide in the evaluation and management of asthma.
Asthma is a chronic inflammatory disorder of the airways that results physiologically in bronchial hyperreactivity, and clinically in recurrent episodes of wheezing, chest tightness or coughing. It is a heterogeneous disease with distinct mechanistic pathways (endotypes) and variable clinical presentations (phenotypes). Regarding endotypes, we can classify cases of asthma according to levels of type 2 (T2) inflammation:
This dichotomy has shaped our thinking about the pathobiology and biochemistry of asthma. Type 2 cytokines (IL-4, IL-5 and IL-13) are produced by CD4-positive T cells; IL-5 is also produced by CD8-positive T cells and natural killer cells, and IL-13 is also produced by innate lymphoid cells.1 IL-4 also increases IL-13 production, which contributes to the physiologic features of asthma such as mucus production, airway fibrosis and hyperresponsiveness.2
Advertisement
Nitric oxide in exhaled breath is produced in the airways by nitric oxide synthase. The inducible isoform of nitric oxide synthase is a mediator of eosinophilic airway inflammation. In T2-high asthma, IL-13 upregulates inducible nitric oxide synthase, leading to increased nitric oxide production. This increased nitric oxide production worsens type 2 inflammation and contributes to airway remodeling and narrowing.3 In people with asthma, the amount of nitric oxide in the breath is proportionate to the number of eosinophils in the sputum, peak flow variability, and hyper-responsiveness to methacholine, and it is reduced by both inhaled and oral corticosteroids.4,5
In 2007, Suresh et al6 performed the first known direct measurement of nitric oxide released from human bronchial epithelial cells. They demonstrated that stimulation with IL-13 results in a significant increase in nitric oxide production by inducing inducible nitric oxide synthase and alters nitric oxide metabolism, resulting in an increase in the amount of nitrate relative to nitrite (see Figure 1). They concluded that the bronchial epithelium is the likely source of nitric oxide in the exhaled breath, and that increased levels observed in inflammatory diseases such as asthma are likely due to inducible nitric oxide synthase upregulation.6 Notably, other inflammatory cytokines such as IL-1-beta and tumor necrosis factor alpha can also upregulate inducible nitric oxide synthase and subsequently increase exhaled nitric oxide, reducing the specificity of nitric oxide for type 2 cytokines such as IL-13.7
Advertisement
In 1998, Dweik et al8 showed that oxygen regulates nitric oxide levels through effects on the kinetics of nitric oxide synthase and proposed that nitric oxide synthase is a mediator of the vascular response to oxygen in the lung.
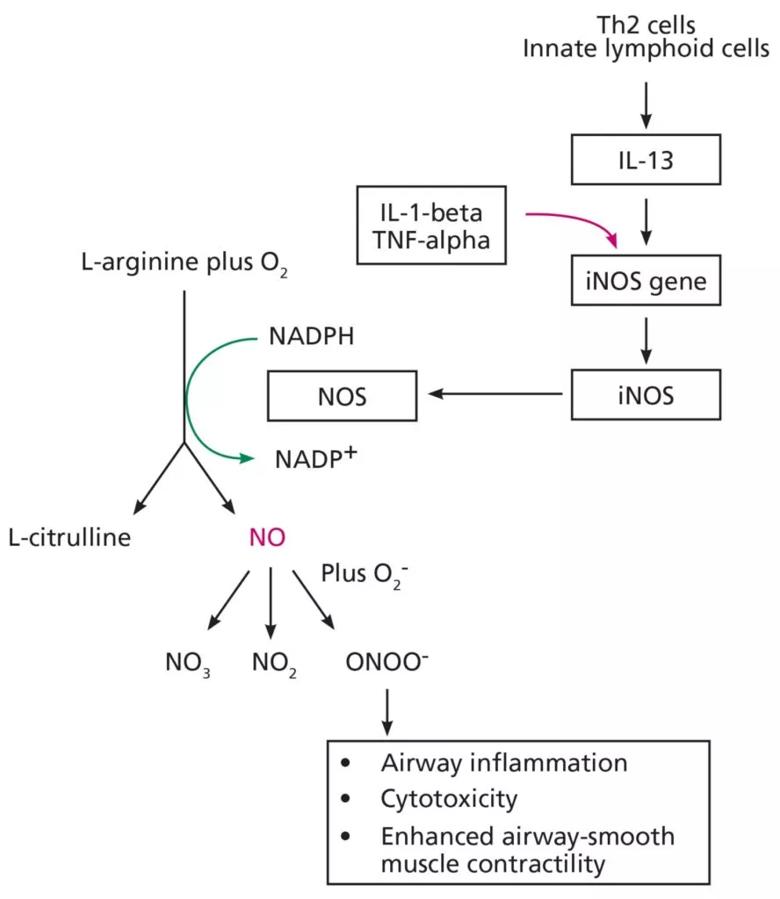
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b79a2683-b007-42db-9389-699a0b88789c/23-PUL-4172458-Fig-1-1-888x1024_jpg)
Figure 1
Asthma is typically diagnosed clinically, but this can sometimes be challenging because asthma is episodic and lacks a gold standard diagnostic test. Numerous studies have evaluated the diagnostic accuracy of measuring exhaled nitric oxide compared with established diagnostic standards such as bronchial provocation, postbronchodilator forced expiratory volume in 1 second, peak-flow variability or a combination of these.9
Karrasch and colleagues9 performed a systematic review and meta-analysis of 26 such studies and reported that nitric oxide testing had an overall 65% sensitivity (95% confidence interval [CI] 58%–72%) and 82% specificity (95% CI 76%–86%) for diagnosing asthma. Higher cutoff values were more specific, while there was no association with sensitivity.
However, confounding factors that increase or decrease nitric oxide need to be considered. Values can be elevated by chronic rhinosinusitis, nasal polyposis, atopy without other features of asthma, rhinovirus respiratory infections, exposure to air impurities, or in patients who are male, older, or taller.10,11 Conversely, factors that can reduce exhaled nitric oxide include cigarette smoking, inhaled corticosteroid use, alcohol use, strenuous exercise and drugs such as leukotriene receptor antagonists and prostaglandins.11
Advertisement
Nitric oxide testing increases the accuracy of asthma diagnosis and is most reliable in patients who are not taking corticosteroids.12 Elevated nitric oxide levels may complement the clinical history and spirometry testing to support the diagnosis of asthma, particularly when clinical suspicion is high. Conversely, low levels can help exclude asthma in the setting of normal spirometry and no suggestive symptoms.
A systematic review of 32 studies (24 in adults, eight in children) concluded that nitric oxide measurement, bronchodilator reversibility, blood eosinophils or immunoglobulin E should not be used individually to diagnose asthma, since using them as stand-alone tests has limited accuracy.13 Therefore, expert opinion is that nitric oxide measurement should be used in conjunction with testing for variable airflow limitation to support the diagnosis of asthma.14
The National Asthma Education and Prevention Program15 recommends that if nitric oxide is measured, it should be done as part of an ongoing monitoring and management strategy. This group also makes a conditional recommendation that if the diagnosis of asthma is uncertain, one can measure nitric oxide as an adjunct to the evaluation. Although there is limited evidence for an exact cutoff point, this group notes that a fractional concentration of exhaled nitric oxide (FeNO) higher than 50 ppb is consistent with type 2 inflammation15 and supports a diagnosis of asthma (other guidelines use a cutoff point of 40 ppb),16,17 whereas a concentration lower than 25 ppb suggests a diagnosis other than asthma.
Advertisement
The British Thoracic Society and the Global Initiative for Asthma favor measuring nitric oxide as an adjunctive tool to diagnose type 2 inflammation18 or to support starting an inhaled corticosteroid.19 The Global Initiative for Asthma highlights the limitation of confounding features of testing—i.e., exhaled nitric oxide can be elevated in nonasthmatic conditions like eosinophilic bronchitis, atopy, allergic rhinitis and eczema and may be normal in T2-low asthma.
The European Respiratory Society20 recommends that if the diagnosis is not clear based on initial bronchodilator reversibility testing, nitric oxide should be measured as part of the diagnostic workup in adults over age 18. A cutoff point of 50 ppb has a high specificity (> 90%) and supports a diagnosis of asthma, but high exhaled nitric oxide levels themselves do not define asthma, and conversely, a value lower than 40 ppb does not rule out asthma.20 FeNO is also low during bronchoconstriction and the early phases of the allergic response and can be variable during viral respiratory infections.
In conclusion, exhaled nitric oxide measurement should not be used in isolation, and current guidelines emphasize the importance of incorporating clinical history, physical examination, and spirometry testing when it is used.12 Its overall specificity is higher than its sensitivity, which indicates it is more useful for ruling in than for ruling out the diagnosis of asthma.
Managing asthma requires addressing environmental factors, ensuring adherence and understanding of the disorder in patients, and partnering with them on goals of care and quality of life. Guidelines recommend increasing the dose of corticosteroids as needed to control symptoms and reduce exacerbations. However, this approach can often lead to patients being on a high dose of corticosteroids without completely attaining benefits.
Like eosinophils in the sputum, nitric oxide in the breath is a good predictor of response to corticosteroid treatment.21 Smith et al21 showed that in patients with respiratory symptoms, especially asthma, those with values higher than 47 ppb had a higher likelihood of responding to steroids. High nitric oxide is a better predictor of steroid responsiveness than bronchodilator reversibility, peak flow variability or airway hyperresponsiveness.21-23 Patients with high nitric oxide levels who have never taken steroids have a better clinical response to them, manifested as improved symptoms and lung function.21
Exhaled nitric oxide is reduced by inhaled corticosteroid therapy, but the magnitude of reduction does not necessarily correlate with clinical response.24 Nitric oxide levels can also help to guide step-down treatment with inhaled corticosteroids. A meta-analysis revealed that in patients on an inhaled corticosteroid and with FeNO levels less than 50 ppb, reducing the dose gradually did not lead to increased exacerbations. However, reducing the dosage in patients with an FeNO of 50 ppb or higher did lead to increased exacerbations.25 Generally speaking, for those on inhaled corticosteroids, a high FeNO does not necessarily suggest a benefit from inhaled corticosteroids, but a low FeNO suggests that increasing the inhaled corticosteroid dose may not be useful (see Figure 2).11,26
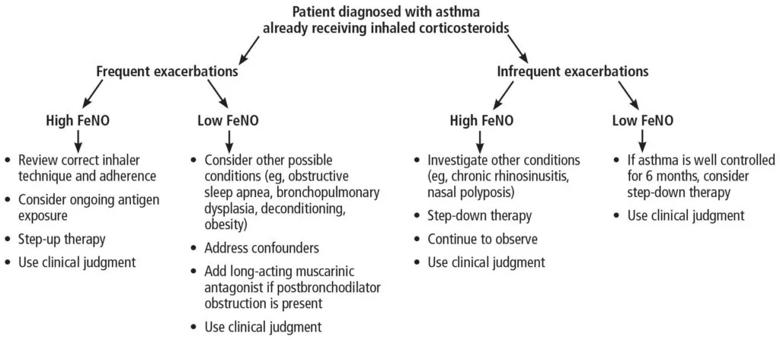
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a60e8f22-0190-444e-9cee-99932547afe2/23-PUL-4172458-Fig-2-1024x447_jpg)
Figure 2
The risk of exacerbations also seems to correlate with a higher FeNO in patients with severe asthma. Kupczyk et al27 found that patients with a baseline FeNO higher than 45 ppb had a rate of exacerbations per year nearly six times higher than those with a lower FeNO.27 Lehtimäki et al26 performed a systematic review and reported that patients with lower FeNO values while on inhaled corticosteroid therapy “probably” have a low risk of subsequent exacerbations.
The risk of exacerbations can be predicted more accurately by taking peripheral blood eosinophilia into account along with the nitric oxide level. Soma et al28 found that patients with eosinophil counts of 0.3 × 109/L or higher and FeNO of 25 ppb or higher were more likely to have an exacerbation compared with those with low levels of both.
Data conflict on exactly how useful nitric oxide testing is in predicting exacerbations in patients with severe asthma. Studies in the United Kingdom showed the Asthma Control Questionnaire was a better predictor of exacerbations than nitric oxide.29 The Liberty Asthma Quest trial30 and a study in a Japanese cohort28 with severe asthma showed that the combination of high blood eosinophil counts and a high FeNO could identify patients prone to frequent exacerbations.
In short, measuring nitric oxide has both prognostic and therapeutic value. The National Asthma Education and Prevention Program15 emphasizes that although nitric oxide measurement can be used in choosing, monitoring and adjusting steroid therapy, it should be an adjunct to other management and monitoring strategies.
In 2014, the definition of severe asthma was updated to distinguish between difficult-to-treat asthma and severe asthma.31 Asthma is called “difficult to treat” if it remains uncontrolled despite treatment with high-dose inhaled corticosteroids or other controller medications or requires this level of treatment to remain well controlled. It is called “severe” if it requires treatment with high doses of an inhaled corticosteroid plus a second controller, systemic corticosteroids or both to prevent it from becoming uncontrolled, or if it remains uncontrolled despite this therapy. Up to 17% of asthma cases are classified as difficult to treat, and 4% to 8% are considered severe.32
Some asthma patients who are prescribed appropriate inhaler therapy and still experience frequent exacerbations are not using their inhalers or are not using them properly. Failure to recognize nonadherence or improper inhaler technique can lead to a vicious cycle of dose escalation and systemic steroid prescriptions, thus leading to potentially avoidable adverse effects of steroid therapy.
Nonadherence to inhaled corticosteroid therapy can contribute to poor asthma control but is sometimes challenging to prove. Measuring nitric oxide after directly observed inhaled corticosteroid administration can serve as an objective method to find out whether patients with difficult-to-treat asthma are adhering to treatment.
In a study in 28 patients with mild asthma, Kharitonov et al33 found that nitric oxide levels decreased within a few days of starting inhaled corticosteroid therapy and increased again after steroid withdrawal.
McNicholl et al34 recruited 22 patients with difficult-to-treat asthma and an FeNO higher than 45 ppb and classified them as adherent or nonadherent based on whether they had filled more than 80% or less than 50% of their prescriptions. After seven days of directly observed inhaled corticosteroid therapy, the nonadherent patients had a greater reduction in FeNO, to 47% vs 79% of their baseline values (P = .003). However, the authors calculated that a five-day test would work nearly as well as a seven-day test (area under the receiver-operating curve 0.86 vs 0.88). They validated the five-day test in a larger group of 40 patients using a 42% or greater drop in FeNO as the number to detect nonadherence. Compared with prescription-filling records and plasma steroid levels, the nitric oxide test demonstrated reasonable discriminatory ability (sensitivity 0.67 [95% CI 0.44–0.84] and specificity 0.95 [95% CI 0.78–0.99]).34
Therefore, nitric oxide suppression testing may have a role in identifying patients who are not adhering to inhaled corticosteroid therapy and, in particular, those who fill their prescriptions but do not actually take the medication.35,36
Devices for measuring FeNO at home have been developed, such as the Niox VERO. A study by Heaney et al37 showed that using these devices during maintenance inhaled corticosteroid therapy resulted in significant reductions in FeNO and blood eosinophil counts and increases in forced expiratory volume in 1 second and asthma-control questionnaire scores.37
Thus, nitric oxide suppression testing may identify patients who may have been labeled as having difficult-to-control asthma but who were not receiving inhaled corticosteroid therapy (due to either nonadherence or poor inhaler technique). These patients may thus be spared escalating steroid doses and subsequent side effects. However, since evidence is sparse in patients without severe asthma, the National Asthma Education and Prevention Program recommends against using nitric oxide as a measure of adherence in them, and the role of nitric oxide suppression for checking adherence is limited to patients with severe asthma.15
Most studies have used peripheral eosinophilia to characterize the T2-high asthma endotype, but elevated exhaled nitric oxide can also serve as an indicator of a T2-high endotype. Consistent with this, elevated nitric oxide has been shown to predict a favorable response to biologic therapies, and low nitric oxide has been shown to predict less improvement with biologic therapy.38 This ability to predict response may confer a cost-saving benefit.39
Notably, in a post hoc analysis, patients with elevated nitric oxide (FeNO ≤ 25 ppb) and peripheral eosinophilia (eosinophil counts > 0.150 × 109/L) had a greater reduction in exacerbations with mepo-lizumab than those with peripheral eosinophilia alone.40 Patients with high FeNO also derived greater benefit from tezepelumab, although those with an FeNO lower than 25 ppb did have a reduction in exacerbations.41 McDowell et al,42 in a prospective observational study, showed that the exhaled nitric oxide level during an exacerbation is useful in discriminating between eosinophilic and noneosinophilic exacerbations in patients treated with mepolizumab. They suggested that nitric oxide be measured during exacerbations and that if FeNO is low (≤ 20 ppb), oral steroids may be of limited utility and antibiotics alone should be considered. Conversely, a high FeNO (≥ 50 ppb) provides support for giving oral steroids.42
The level of exhaled nitric oxide should be combined with other measures to assess asthma control and should be interpreted within the context of the pretest probability.43,44 If there are no confounding factors, a low level of exhaled nitric oxide suggests that a response to corticosteroids is unlikely, whereas a high level suggests that a response to corticosteroids is likely. Ultimately, however, it is the individual clinician who identifies persistent inflammation and decides on initiating or stepping down corticosteroid therapy.
In summary, exhaled nitric oxide should not be the sole tool used to diagnose asthma, although when used in the right clinical context, it can support the diagnosis of asthma, predict the response to inhaled corticosteroid therapy, monitor compliance, and predict the prognosis of asthma.
REFERENCES
Advertisement
Largest study examines factors affecting asthma exacerbations during and after pregnancy
New research classifies asthma into five clinically important subphenotypes
Findings illuminate MCEMP1 protein’s role in severe inflammation
New breakthroughs are shaping the future of COPD management and offering hope for challenging cases
New research adds to understanding of an understudied link
Takeaways from the most recent annual meeting centered around clinical advances, AI integration and professional development
Recent breakthroughs have brought attention to a previously overlooked condition
A review of treatment options for patients who may not qualify for surgery