Accuracy does not equal clinical utility

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6af03c5f-493f-4527-822c-a2b4ece450c1/GettyImages-687876154-jpg)
Microscope
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
As clinicians continue to weigh the risks and benefits of lung cancer screening with their patients, and we come closer to standardized screening models, the promise of reducing harm through molecular biomarker prediction and detection is certainly appealing. However, we must take great care in the evaluation of these biomarkers to avoid confusing accuracy with clinical utility.
To that end, the American Thoracic Society (ATS) Assembly on Thoracic Oncology developed a policy statement detailing several important considerations:
Biomarkers may be considered accurate at 90 percent sensitivity and 90 percent specificity, but with the calculations provided in the guidelines, may still prove to hold great potential for harm. We thus provide detailed formulas to calculate the minimum accuracy of a biomarker to warrant further study.
Another important aspect of clinical utility is the ability of the biomarker to add predictably to a physician’s judgment and thus produce a better clinical outcome.
For lung cancer screening, a molecular biomarker must cause:
Advertisement
For lung nodule management, a molecular biomarker is considered clinically useful when:
In order to determine clinical utility of a biomarker for lung cancer screening, we recommend the following four study designs:
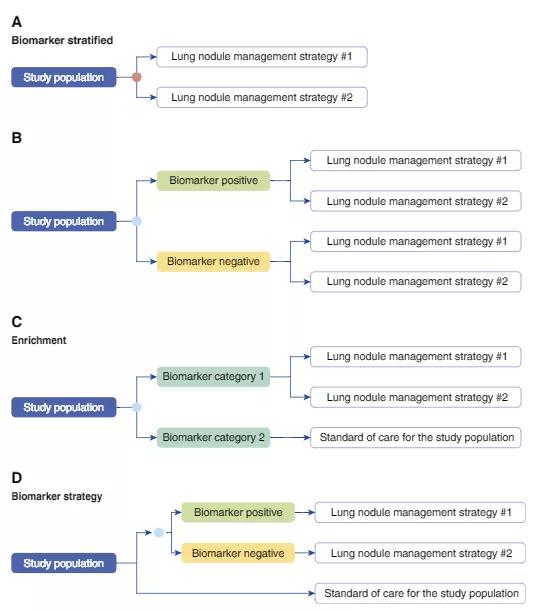
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f5534a0c-54fb-4abf-b2b4-40e387487dc4/Mazzone-inset_png)
Caption: Examples of study designs capable of assessing the study outcomes of interest in a trial assessing the clinical utility of a lung cancer screening molecular biomarker. “Lung cancer screening intervention” may represent low-dose computed tomography screening, no screening, standard of care for the population, or another screening test. Biomarker stratified: (A) Biomarker (orange circle) is measured retrospectively from prospectively collected archived samples, or is measured prospectively, but the results are not used to determine the study arm. (B) Biomarker (blue circle) is measured prospectively, and the result is used to stratify patients. Ideally, both biomarker-positive and biomarker-negative patients are randomized to one of the screening interventions. Enrichment: (C) In populations at very low risk of having lung cancer it may not be practical to randomize those in the biomarker-negative arm to a screening intervention other than standard of care for the studied population. Biomarker strategy: (D) The study population is randomized to a biomarker (blue circle)–strategy arm, where the receipt of the screening intervention is based on the biomarker results, and an arm where all receive standard of care for the study population. Reprinted with permission of the American Thoracic Society. Copyright © 2018 American Thoracic Society. Mazzone PJ, Sears CR, Arenberg DA et al; on behalf of the ATS Assembly on Thoracic Oncology. Evaluating molecular biomarkers for the early detection of lung cancer: when is a biomarker ready for clinical use? An official American Thoracic Society policy statement. AJRCCM. 2017;196(7):e15-e29. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Advertisement
We created a similar flowchart for lung nodule management, available in the full policy statement. In short, studies should be large, well designed, expertly conducted and capable of measuring biomarker testing’s impact on a clinician’s decisions and patients’ outcomes, in order to meet ATS guidelines.
One of the more nuanced and difficult questions with which the committee grappled was the difference between biomarkers determining cancer risk and those detecting cancer early. After considering questions about the appropriate time interval between testing and diagnosis to categorize prediction versus detection biomarkers, and using risk stratifications to classify biomarkers, we determined that mandating single, standardized definitions was not the appropriate path. Instead, studies should take care to clearly define biomarkers as intended for risk prediction or early cancer detection. Similarly, we did not establish a standard definition of early detection of lung cancer, and concluded that studies should also determine those parameters and describe them clearly. In terms of clinical utility, an early detection biomarker must discover lung cancer that benefits substantially from early detection and treatment.
For the evaluation of lung nodules, a clinically useful molecular biomarker could expedite therapy for patients with malignancies and reduce aggressive interventions for patients without malignancies. Biomarkers in the diagnosis and evaluation category must improve on the accuracy of currently available lung nodule risk calculators, fludeoxyglucose F 18-PET imaging and/or clinical judgment in order to warrant further study.
Advertisement
The potential for molecular biomarkers to tip the balance from benefit to harm in lung cancer screening and nodule diagnosis and to enhance risk prediction warrants a robust discussion about how we determine their accuracy and readiness for clinical use. These guidelines offer a suggested course for those of us invested in the more rapid and accurate and less harmful prediction, detection and diagnosis of lung cancer.
Dr. Mazzone is Director of the Lung Cancer Program and Lung Cancer Screening Program for the Respiratory Institute.
Advertisement
Advertisement
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches