Pairing low-frequency ultrasound with microbubble resonators to penetrate the blood-brain barrier
Glioblastoma — the most common malignant brain tumor among adults — is also one of the most therapeutically elusive. “One of the main challenges is that chemotherapies have trouble penetrating the blood-brain barrier,” notes Glen Stevens, DO, PhD, neuro-oncologist in Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center. “As a result, we are always looking at new techniques that can allow us to get drugs into the brain.”
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A novel approach currently under investigation involves the use of focused ultrasound therapy with microbubbles to disrupt the blood-brain barrier so that chemotherapy can reach the area containing tumor cells. Unlike high-frequency ultrasound, which is commonly used to treat patients with tremor, the Exablate Model 4000 Type 2 system uses low-frequency ultrasound, which is often more tolerable to patients.
“This transcranial, incisionless treatment combines low-frequency ultrasound with focused microbubble resonators,” explains Dr. Stevens. “We use a product called Definity, which uses mechanical oscillation of the microbubbles to temporarily disrupt the blood-brain barrier. This can all be done in real time, allowing us to control the opening of the barrier and other variables.”
To better understand the potential of this treatment, a phase 1/2 single-arm study was initiated. Cleveland Clinic is one of several U.S. centers participating in the prospective trial.
The study is designed to evaluate the safety and feasibility of blood-brain barrier disruption in combination with intravenous carboplatin for treatment of recurrent glioblastoma using the Exablate system with microbubble resonators.
Adults with recurrent glioblastoma who plan to undergo carboplatin monotherapy are eligible; patients with multiple recurrences may be included. Key exclusion criteria are prior toxicity with carboplatin chemotherapy, previous treatment with an anti-VEGF agent such as bevacizumab, and cerebellar spinal cord or brain stem tumors.
Advertisement
Enrolled patients receive Exablate blood-brain barrier disruption combined with chemotherapy. Intravenous carboplatin is administered every four weeks for up to six cycles. Before administration of carboplatin, participants undergo the Exablate procedure to open the blood-brain barrier in the targeted areas.
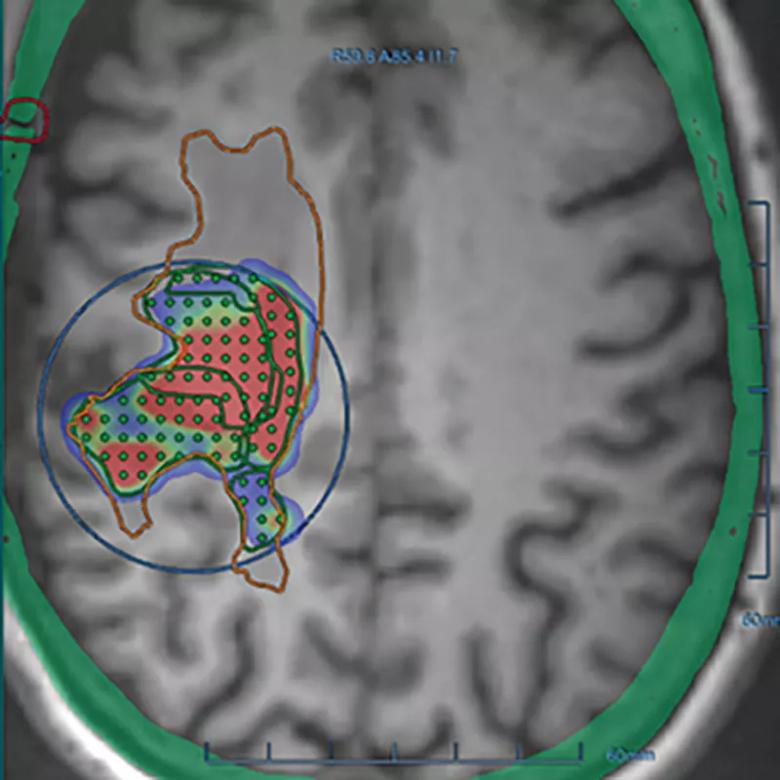
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/81190d91-9063-4f2f-b2a2-ea8e2dba8f62/21-NEU-2248958-CQD-Inset-800x800-1_jpg)
Figure. A localizing T1 MPRAGE volumetric axial MRI from a patient in the study. The orange line outlines the infiltrating FLAIR signal associated with the tumor. The blue circle is the transducer that outlines a region that can undergo disruption of the blood-brain barrier (BBB). The dots or subspots are grouped into areas of 32 and define an area that will undergo disruption. The green, blue and red areas within the transducer field show real-time BBB disruption.
The estimated study completion date is November 2023 with an accrual goal of 30 patients across all centers. As of July, the Cleveland Clinic team — led by Dr. Stevens — had treated two patients with four rounds of therapy each.
With a focus on safety and feasibility, the researchers are monitoring patients closely for adverse events. This includes conducting imaging after each treatment to exclude issues such as bleeding or inflammation. “Ultimately, this study will shed light on any potential adverse events related to this treatment approach,” Dr. Stevens notes.
While still in its infancy, the trial has highlighted the promise of this approach. The researchers have successfully used the Exablate system with microbubble resonators to open the blood-brain barrier. The barrier is estimated to remain open for approximately 24 hours; patients are infused with carboplatin within four hours of Exablate therapy.
Advertisement
Since steroids can act to close the blood-brain barrier, the researchers have been careful to use no more than 2 mg of dexamethasone when needed. To date, Dr. Stevens and his team have successfully managed patients with either no steroids or a low dose.
Both the Exablate treatment and carboplatin have been well-tolerated by the patients at Cleveland Clinic, and no significant adverse events have been observed. “This is a significant hurdle to overcome,” Dr. Stevens says. He adds that because the procedure takes place with the patient within an MRI scanner, his neuroradiologist colleagues Daniel Lockwood, MD, and Emmanuel Obusez, MD, have been instrumental in offering the procedure. “It is not easy to lie in a machine for several hours with a head frame on, but our patients have been able to tolerate it well, which I find encouraging,” Dr. Stevens says.
He is hopeful this study will serve as a proof-of-principle investigation and pave the way for a novel approach that finally addresses the long-standing blood-brain barrier challenge in glioblastoma. If proven feasible, he says, the approach could have implications for other neurodegenerative disorders.
“Even as an early clinical trial, we are offering patients access to a novel approach that goes beyond standard of care,” Dr. Stevens notes, while emphasizing the importance of ongoing discovery. “Glioblastoma is a challenging disease with a difficult prognosis, but there are innovative trials ongoing in the field. We are not standing still or moving backward. Not all trials are for all patients, but the more trials we have, the more options we can offer our patients.”
Advertisement
Advertisement

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units

Addressing rare disease and challenging treatment course in an active young patient