New findings about the drug’s safety and efficacy from a phase II trial

The small molecule ONC201 has shown promise as an effective, well-tolerated drug in the treatment of neuroendocrine tumors (NETs), report the authors of a new study published in Clinical Cancer Research.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Peter Anderson, MD, PhD, principal investigator of the study and pediatric oncologist at Cleveland Clinic Children’s, presented the initial findings from the phase II trial at the 2021 American Society of Clinical Oncology meeting.
These findings demonstrated that weekly treatment with ONC201 was associated with partial response or stable disease in nearly all patients with two different types of neuroendocrine tumors: pheochromocytoma-paraganglioma (PC-PG) and desmoplastic small round cell tumor (DSRCT).
There is a need for tolerable treatment to control disease without hindering patients’ quality of life. ONC201 provided maintenance of performance status with development of few new metastases. While this drug is not the only treatment option for NETs, this well-tolerated treatment can be combined with other medications.
The primary endpoint of the phase II trial of ONC201 was radiographic response using RECIST criteria. Secondary endpoints included progression-free survival, overall survival, and safety.
Thirty patients were enrolled in this investigator-initiated trial (NCT03034200). Arm A (n = 10), which included patients with metastatic PC-PG, and Arm B (n = 12), which included patients with other NETs, each received a 625 mg weekly oral dose of ONC201 for three months or longer and until progression.
Advertisement
Dr. Anderson explains, “To our surprise, almost all of the patients in Arm C, the more dose-intense regimen, had some response, according to the RECIST criteria. Even though it’s twice as much drug, we didn’t see twice as much toxicity. We saw exactly the same toxicity.”
Importantly, disease progression was mainly noted in tumor growth and not in the form of new lesions. However, because no complete responses were observed with either regimen (once versus twice weekly), the authors suggest ONC201 may be more effective as combination therapy or an adjuvant option to maintain performance status while having metastatic disease.
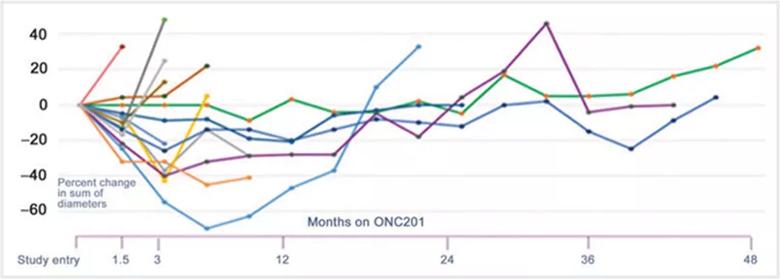
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/53fe08a5-b9e7-482c-8bbf-5d5ff0901f26/22-CHP-2649111-CQDAnderson-Neuroendocrine-Tumors-Study-805x287-1_jpg)
Figure. Graph depicting PC-PG tumor responses depicted over time. Dr. Anderson says presenting the data in this way is important to understand responses over time.
This figure was reproduced under the terms of the Creative Commons CC BY license; it originally appeared in: Peter M. Anderson, Matteo M. Trucco, Rohinton S. Tarapore, Stacey Zahler, Stefanie Thomas, Janette Gortz, Omar Mian, Martin Stoignew, Varun Prabhu, Sara Morrow, Joshua E. Allen; Phase II Study of ONC201 in Neuroendocrine Tumors including Pheochromocytoma-Paraganglioma and Desmoplastic Small Round Cell Tumor. Clin Cancer Res 2022; https://doi.org/10.1158/1078-0432.CCR-21-4030
Aside from the PC-PG and DSRCT cohorts, investigators were not able to draw meaningful conclusions from outcomes associated with other NETs, which included medullary thyroid carcinoma, adrenocortical carcinoma, cholangiocarcinoma, and clear cell sarcoma.
Advertisement
Given the relative rarity of NETs and the heterogeneity of the patient population, Dr. Anderson says a multi-institutional trial or study designs using patients as their own controls, may be warranted in the future to improve the estimates for response rates and duration of therapy.
The data are important, nonetheless, particularly as scientists continue to understand specific mutation drivers in the tumor microenvironment and develop personalized immune therapies for patients with rare cancers.
“There are many other imipridones in development and more research is needed to determine whether ONC201 is the optimal treatment; however, this study shows it has a favorable and tolerable profile in patients with PC-PG and DSRCT,” he concludes.
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors