The road to FDA approval and what's ahead
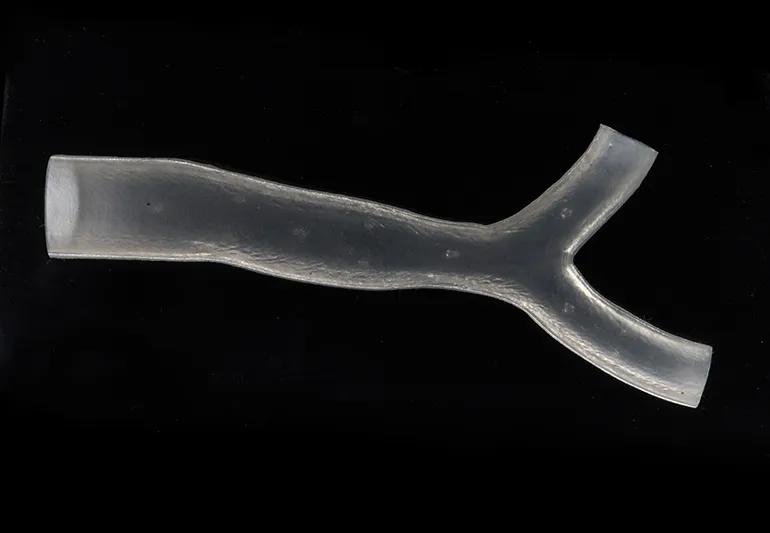
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/47b3ae5e-94c2-4303-8d6e-11e5cea08668/3D-Printed-Airway-Stent-jpg)
650×450-lung-stent
In late 2019, the U.S. Food and Drug Administration (FDA) cleared the use of 3D-printed patient-specific airway stents to treat patients with complex breathing disorders. Unlike traditional airway stents that are retrofitted (standard sizes) for a patient’s anatomy, these stents are created specifically for the patient.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Thomas Gildea, MD, the pulmonologist who pioneered this innovation, notes that the basis of this project stemmed from treating patients who were experiencing complications with existing stents. “Even in the best circumstances, when we saw outcomes improve, there were still times I couldn’t make exactly what I wanted,” says Dr. Gildea. “I knew we could do better.”
The use of standard silicone stents as an intervention for breathing disorders has been widely practiced and reported in the literature for decades. Silicone Y-stents, which are designed to be modified for patients, have been available commercially for years.
These stents come in a limited number of sizes and shapes and are generally designed for larger airways. However, no two patient anatomies and diseases are alike, making it difficult to get a perfect fit, especially for those with complex conditions. Even in parts of the airways that are easily accessible, ill-fitting standard stents can result in stent kinking and bending as well as airway complications such as growth of new tissue, mucous impaction and tissue death.
Dr. Gildea says it made sense to use the patient’s anatomy as a guide. The patient-specific stents are designed using CT scans and proprietary 3D visualization software. The molds for the stents are then printed using a 3D printer and injected with medical-grade silicone. This process allows them to perfectly fit a patient’s anatomy.
Another advantage of the patient-specific silicone stents is they have the potential to be more tolerable than traditional silicone stents, which, in certain patients, may have to be frequently changed or cleaned due to problems from a poor fit. In studies, the patient-specific stents lasted, on average, about a year versus 90 days for stock stents. Furthermore, the patient-specific stents exhibited shorter procedure times and improved patient-reported symptoms, leading to a reduced need for stent changes and modifications.
Advertisement
“It’s been a five-year process,” says Dr. Gildea. “We were fortunate enough to have received funding mechanisms through the NIH Centers for Accelerated Innovations, the innovator acceleration grant that is co-sponsored by Cleveland Clinic. This helped jumpstart some of the work early on in the process.”
This recent FDA clearance follows several years of regulatory work and laboratory-based research designed to show an equivalence between traditional stents and patient-specific stents. The team also spent considerable time on software development and training to standardize the process and create a seamless experience for physician use.
Until now, the patient-specific devices were being implanted under the FDA’s compassionate use program, which allows patients who have failed all available forms of treatment to receive investigational ones not yet available to the public.
Dr. Gildea says the ideal candidate is someone with complex, chronic airway problems. The FDA clearance of the device makes it more accessible to patients whose breathing has been compromised due to surgical complications related to lung transplant or tumor resection or other diseases that cause airway obstructions.
Procedurally, there are few differences in how traditional stents and 3D-printed stents are placed in patients. “It’s like putting any other silicone stent in, except that this one was made for the patient,” says Dr. Gildea. Typically, this is a same-day procedure, whereby patients undergo general anesthesia, and physicians use a rigid bronchoscope to prepare the airway for stent placement. Once the stent is deployed, it usually requires a mild-to-moderate amount of repositioning to fit the patient just right.
Advertisement
A new subsidiary named VisionAir Solutions will be formed around the technology with the sole mission of bringing more personalized medical devices to interventional pulmonologists and the patients who need them. By the end of the first quarter of 2020, this new spin-off company plans to begin providing the personalized stents to patients at Cleveland Clinic and University Hospitals.
And there is more work to be done, adds Dr. Gildea, who says that the team will continue to expand on the device and enhance the software for possible other uses such as diagnosis.
Longer-term goals for product development include the continued investigation into appropriate sizing. For example, patients may respond dramatically when the stent is first placed and local inflammation is resolved but then may require a newly sized stent. In addition to sizing, there may be an opportunity to explore the material science aspect of the program in the future.
For now, the team is chiefly concerned with how to make the product available to physicians — and ultimately to patients. “Everyone in interventional pulmonology has seen, or currently has, that patient who may stand to benefit from this. We are working hard to make this technology more widely available.”
Advertisement
Advertisement
Takeaways from the most recent annual meeting centered around clinical advances, AI integration and professional development
Recent breakthroughs have brought attention to a previously overlooked condition
A review of treatment options for patients who may not qualify for surgery
Looking at the real-world impact and the future pipeline of targeted therapies
The progressive training program aims to help clinicians improve patient care
New breakthroughs are shaping the future of COPD management and offering hope for challenging cases
Exploring the impact of chronic cough from daily life to innovative medical solutions
How Cleveland Clinic transformed a single ultrasound machine into a cutting-edge, hospital-wide POCUS program