Urine is the window to the kidney
By James F. Simon, MD, and Arani Nanavati, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The urine is the window to the kidney. This oft-repeated adage impresses upon medical students and residents the importance of urine microscopy in the evaluation of patients with renal disorders.
While this phrase is likely an adaptation of the idea in ancient times that the urine reflected on humors or the quality of the soul, the understanding of the relevance of urine findings to the state of the kidneys likely rests with the pioneers of urine microscopy. As reviewed by Fogazzi and Cameron, the origins of direct inspection of urine under a microscope lie in the 17th century, with industrious physicians who used rudimentary microscopes to identify basic structures in the urine and correlated them to clinical presentations. At first, only larger structures could be seen, mostly crystals in patients with nephrolithiasis. As microscopes advanced, smaller structures such as “corpuscles” and “cylinders” could be seen that described cells and casts.
In correlating these findings to patient presentations, a rudimentary understanding of renal pathology evolved long before the advent of the kidney biopsy. Lipid droplets were seen in patients swollen from dropsy, and later known to have nephrotic syndromes. In 1872, Harley first described the altered morphology of dysmorphic red blood cells in patients with Bright disease or glomerulonephritis.
In 1979, Birch and Fairley recognized that the presence of acanthocytes differentiated glomerular from nonglomerular hematuria.
The term dysmorphic refers to any misshapen red blood cell found in the urine. Dysmorphic cells have a variety of causes. The term acanthocyte is reserved for red blood cells that show evidence of damage thought to be induced by passage through the glomerular basement membrane, characterized by vesicle-shaped protrusions or blebs (Figure 1). These cells are considered quite specific for glomerular hematuria. Köhler et al found that in patients with biopsy-proven glomerular disease, 12.4 percent of excreted cells were acanthocytes, whereas they were rarely found in people with nonglomerular hematuria. As these cells then pass through the renal tubules, they can become encased in Tamm-Horsfall proteins, forming red blood cell casts (Figure 2), another hallmark of glomerular disease.
Advertisement
The kidney biopsy from a patient with immunoglobulin A nephropathy presented by Daza et al (Cleveland Clinic Journal of Medicine, January 2018) reminds us of the amazing pathophysiology of glomerular disease. A red blood cell can somehow contort enough to squeeze through the pores of an inflamed glomerular basement membrane roughly one-tenth its size, with only blebbing to show for it. The image Daza et al present captures this rarely seen event and should give us pause. In an age when the electronic medical record too often replaces the patient history, when ultrasonography and echocardiography are replacing the stethoscope, and when reports by machines and technicians with no understanding of the patient’s condition replace direct examination of bodily fluids, there is merit in seeing what is going on for ourselves. This image allows us to understand the value of urine microscopy in the workup of patients with renal disease.
The tools used in urine microscopy have advanced significantly since its advent. But not all advances have led to improved patient care. Laboratories have trained technicians to perform urine microscopy. Analyzers can identify basic urinary structures using algorithms to compare them against stored reference images.
More important, urine microscopy has been categorized by accreditation and inspection bodies as a “test” rather than a physician-performed competency. As such, definitions of quality in urine microscopy have shifted from the application of urinary findings to the care of the patient to the reproducibility of identifying individual structures in ways that can be documented with quality checks performed by nonclinicians. And since the governing bodies require laboratories to adhere to burdensome procedures to maintain accreditation (e.g., the US Food and Drug Administration’s Clinical Laboratory Improvement Amendments), many hospitals have closed nephrologist-based urine laboratories.
Advertisement
This would be acceptable if laboratory-generated reports provided information equivalent to that obtained by the nephrologist. But such reports rarely include anything beyond the most rudimentary findings. In these reports, the red blood cell is not differentiated as dysmorphic or monomorphic. All casts are granular. Crystals are often the highlight of the report, usually an incidental finding of little relevance. Phase contrast and polarization are never performed.
Despite the poor quality of data provided in these reports, because of increasing regulations and time restrictions, a dwindling number of nephrologists perform urine microscopy even at teaching institutions. In an informal 2009 survey of nephrology fellowship program directors, 79 percent of responding programs relied solely on lab-generated reports for microscopic findings (verbal communication, Perazella, 2017).
There is general concern among medical educators about the surrendering of the physical examination and other techniques to technology. However, in many cases, such changes may improve the ability to make a correct diagnosis. When performed properly, urine microscopy can help determine the need for kidney biopsy, differentiate causes of acute kidney injury, and help guide decisions about therapy. Perazella showed that urine microscopy could reliably differentiate acute tubular necrosis from prerenal azotemia. Further, the severity of findings on urine microscopy has been associated with worse renal outcomes.
Advertisement
At our institution, nephrologist-performed urine microscopy resulted in a change in cause of acute kidney injury in 25 percent of cases and a concrete change in management in 12 percent of patients (unpublished data).
With this in mind, it is concerning that the only evidence in the literature on this topic demonstrated that laboratory-based urine microscopy is actually a hindrance to its underlying purpose in acute kidney injury, which is to help identify the cause of the injury. Tsai et al showed that nephrologists identified the cause of acute kidney injury correctly 90 percent of the time when they performed their own urine microscopy, but this dropped to 23 percent when they relied on a laboratory-generated report. Interestingly, knowing the patient’s clinical history when performing the microscopy was important, as the accuracy was 69 percent when a report of another nephrologist’s microscopy findings was used.
The purpose of urine microscopy in clinical care is to identify and understand the findings as they apply to the patient. When viewed from this perspective, the renal patient is clearly best served when the nephrologist familiar with the case performs urine microscopy, rather than a technician or analyzer in remote parts of the hospital with no connection to the patient.
Advances in technology or streamlining of hospital services do not always produce improvements in patient care, and how we define quality is integral to identifying when this is the case. Quality checklists can serve as guides to safe patient care but should not replace clinical decision-making. Direct physician involvement with our patients has concrete benefits, whether taking a history, performing a physical examination, reviewing radiologic images, or looking at specimens such as urine. It allows us to experience the amazing pathophysiology of human illness and to understand the nuances unique to each of our patients.
Advertisement
But most important, it reinforces the need for the direct bond, both emotional and physical, between us as healers and our patients.
Dr. Simon is in the Department of Nephrology and Hypertension. Dr. Nanavati is Transplant Nephrology Fellow in the Department of Nephrology and Hypertension.
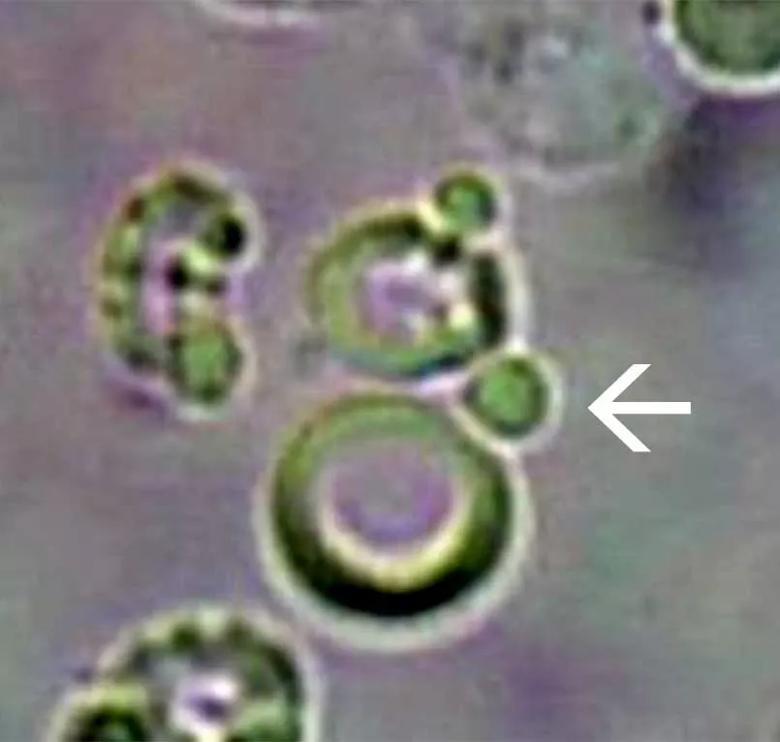
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7fa7a650-5a81-4cce-8e00-b284df1b7502/805x-2-Urine-Micro_jpg)
Figure 1. An acanthocyte seen in a patient with glomerulonephritis. The arrow notes a typical bleb (× 40).
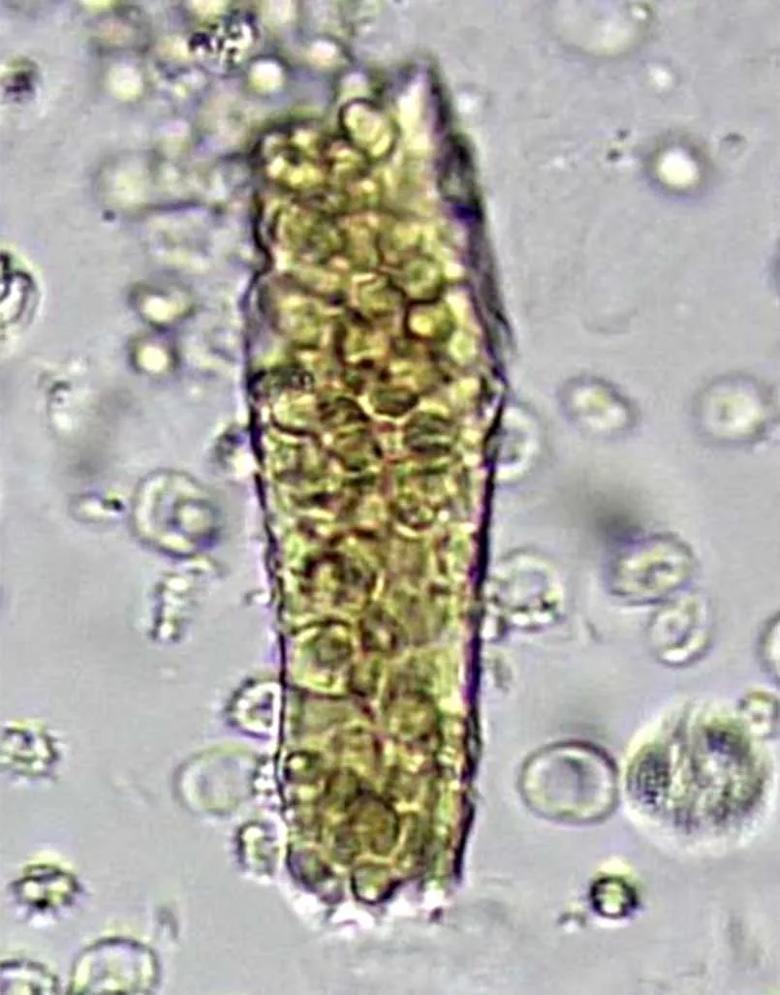
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/473998bf-7c33-4caf-a3dc-8c3e6b731068/805x-Urine-Micro_jpg)
Figure 2. A red blood cell cast in a patient with glomerulonephritis. Casts form when red blood cells that have passed through a damaged glomerular basement membrane are encased in urinary proteins before being excreted into the urine (× 40).
This editorial originally appeared in Cleveland Clinic Journal of Medicine in January 2018 (Vol. 85, No. 1). Read the full article and references, Quality in Urine Microscopy: The Eyes of the Beholder.
Advertisement

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Fixed-dose single-pill combinations and future therapies

Reproductive urologists publish a contemporary review to guide practice

Two recent cases show favorable pain and cosmesis outcomes

Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood

Proteinuria reduction remains the most important treatment target.

IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy

Oncologic and functional outcomes are promising, but selection is key