Real-world results reporting aims to make treatments safer and more effective

Below is an abbreviated version of an article that was originally published by Lerner Research Institute (LRI).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Cleveland Clinic has reported its real-world experience with the new KEYNOTE-522 regimen to treat triple-negative breast cancer in the Annals of Surgical Oncology.
“As one of the first institutes to report our experience with KEYNOTE-522 after the clinical trial, we have a responsibility to share our real-world findings so that treating physicians and patients understand what to expect from this regimen,” says Professor of Surgery, Director of the Eastern Region of Cleveland Clinic’s Breast Cancer Program and Chief of Breast Surgery at Hillcrest Hospital, Julie Lang, MD. Our publication helps breast surgical oncologists and their patients with triple-negative breast cancer with surgical treatment planning by analyzing factors relevant to the surgical care of these patients.”
Triple-negative breast cancer is known to have poor prognosis and high rates of recurrence. KEYNOTE-522 is a new triple-negative breast cancer treatment that combines regular chemotherapy with immunotherapy treatments, prior to tumor removal surgery.
“This regimen causes the cancer cells in the breast to completely respond in many patients with triple negative breast cancer. We suddenly have a regimen that's highly effective, and we had to adjust our approach accordingly,” Dr. Lang says. “However, the clinical trial report didn’t cover everything surgeons would want to know when determining a care plan.”
Dr. Lang and her colleagues questioned whether KEYNOTE-522 treatment influenced the type of surgical treatment their patients qualified for, a factor that wasn’t examined during initial clinical trials.
Advertisement
Dr. Lang and her team looked back at 240 patients who received treatment for triple-negative breast cancer between 2019 and 2022 to identify differences between individuals treated with the KEYNOTE-522 regimen. Women who underwent KEYNOTE-522 had higher survival rates, which aligned with the clinical trial results.
Beyond pure survival, Dr. Lang’s team found that the KEYNOTE-522 regimen shrunk many patients’ tumors to a more manageable size that allowed more options for their care plan. The study found:
• Significantly more women qualified for breast-conserving therapy (a combination of radiation therapy and removing the tumor).
• Many who qualified for breast-conserving therapy still opted for mastectomies and reconstructive surgery.
• Significantly fewer women required lymphadenectomies.
Dr. Lang and her team saw higher rates of hormone-related side effects in Cleveland Clinic’s data than the clinical trial reported. Left untreated, hormone imbalances can cause problems like adrenal crises during surgery.
“At Cleveland Clinic, we continuously monitor hormone levels in our patients receiving immunotherapy treatment,” Dr. Lang explains. “It’s because we monitor hormones that we can catch potential issues for surgery and keep patients safe – but not all hospital systems keep track of these things the same way.”
Dr. Lang still wholeheartedly recommends the KEYNOTE-522 regimen to her patients but says it’s important to share these results widely for the benefit of patient care. “We hope that our findings will help other hospitals design appropriate treatment plans for their own patients as they, too, adopt this new regimen.”
Advertisement
The full-length original version of this article is available at the LRI website.
Advertisement
Advertisement

Obstructing key protein allows for increased treatment uptake for taxane chemotherapy

Polygenic risk score could help predict who will develop this aggressive breast cancer
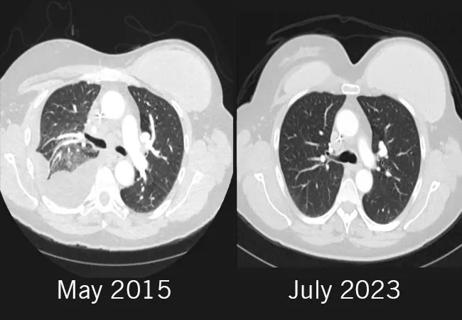
Combination of olaparib and carboplatin results in complete durable response for a patient with BRCA2 and “BRCAness” mutations

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Reconsidering axillary lymph node dissection as well as depth of surgical margins

Ultra-Hypofractionated Whole Breast Irradiation and Partial Breast Irradiation Reduce Many Toxicities

Robotic-Assisted Procedures Offer Breakthroughs in Lymphedema Treatment After Breast Cancer Surgery

Best practices for reducing toxicities