Objective risk assessment guides surveillance and treatment
Patients with Barrett’s esophagus (BE) face a low but consequential risk of progression to esophageal adenocarcinoma, for which treatments are limited. Gastroenterologists need reliable methods to assess progression risk in BE patients — especially those who are nondysplastic — in order to make effective decisions about early therapeutic intervention versus surveillance.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A recently developed systems pathology test effectively identifies nondysplastic BE patients who are at increased progression risk and would benefit from more frequent surveillance and potential intervention, according to results of a Cleveland Clinic-led independent validation study presented at Digestive Disease Week 2019.
Principal Investigator Prashanthi Thota, MD, Medical Director of the Esophageal Center in Cleveland Clinic’s Digestive Disease & Surgery Institute, explains how the findings will help physicians manage BE patients with improved accuracy and utilize treatment resources more effectively.
“Previously, we would have all nondysplastic BE patients continue to return for surveillance endoscopies every 3 to 5 years to check for dysplasia,” Dr. Thota says. “This unique tissue systems pathology test allows us to risk-stratify patients with nondysplastic BE who are at increased risk for progression to high-grade dysplasia and esophageal adenocarcinoma and therefore may benefit from endoscopic eradication therapy.”
“It’s no secret that endoscopic ablation therapy is expensive and not without complications,” says Dr. Thota. “Because of this, physicians must be highly selective when it comes to treating patients with ablation.”
In the past, dysplasia was the single determinant for applying ablation therapy in BE cases, since it signified precancerous changes in the esophagus. However, the majority of patients with BE are not dysplastic, although some of these patients will develop esophageal adenocarcinoma.
Advertisement
In cases where tissue biopsies are performed, malignant cellular changes may not yet be histologically apparent; results also are subject to the random nature of biopsy sampling and varying interpretation by observers.
Accurate risk-stratification tests have been lacking and are necessary to improve outcomes.
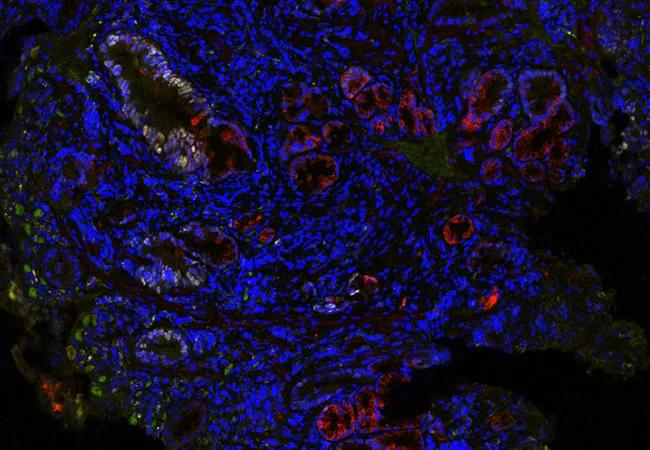
The single-blinded, nested case-control study was conducted to further validate a previously described tissue systems pathology test. The TissueCypher® test utilizes multiplexed immunofluorescence with whole-slide fluorescence imaging and proprietary image analysis to quantify nine protein-based biomarkers and nuclear morphology in biopsy specimens. Quantitative measurements derived from the biomarkers produce a score correlated with the risk of progression to high-grade dysplasia and esophageal adenocarcinoma (HGD/EAC) within five years.
To assess the assay’s accuracy, the researchers compared the results of its risk stratification of BE patients with those patients’ clinical outcomes.
The researchers analyzed 268 BE patients from Cleveland Clinic and the University of Pittsburgh, matching those who progressed to HGD/EAC in more than one year (58) with those who showed no progression after at least five years of surveillance (210).
The standard baseline/pre-progression biopsies of patients who had been graded as either non-dysplastic (ND), indefinite for dysplasia (IND), low-grade dysplasia (LGD) or high-grade dysplasia were tested with TissueCypher in a blinded manner. Dr. Thota collaborated with John Goldblum, MD, the Chair of Cleveland Clinic’s Department of Anatomic Pathology, to collect sections from the formalin-fixed paraffin-embedded biopsies and analyze the results.
Advertisement
The researchers found that the TissueCypher assay accurately stratified BE patients with ND, IND and LGD diagnoses into low-, intermediate- and high-risk classes (hazard ratio 4.7, 95% confidence interval 2.5, 8.8 [high-risk vs. low-risk], p < 0.0001). The test’s prevalence-adjusted positive predictive value at five years was 22.6%, indicating a progression rate of 4.6% per year in patients who TissueCypher scored as high-risk.
The researchers observed the test’s best risk-stratification performance in ND patients. Those ND patients who TissueCypher scored as high-risk progressed at a higher rate than patients with a gastrointestinal subspecialist-confirmed diagnosis of LGD (6.3% per year for ND high-risk vs. 4.4% per year for confirmed LGD).
The ultimate goals of high-accuracy risk assessment are improved care and enhanced outcomes for BE patients. “If we can identify patients without dysplasia who are at higher risk for progression, we can offer them timely interventions that may help prevent esophageal cancer,” Dr. Thota says. “Ablation treatments can be started immediately to keep cancer or dysplasia from developing, thereby extending the patient’s quantity and quality of life.”
The study results — particularly the ability to confidently assess patients as low risk for progression — also impact physician recommendations for BE surveillance. “For patients with a low risk of progression, we may recommend surveillance endoscopies at 5- and 10-year intervals,” says Dr. Thota. “Basically, the lower the risk, the longer the surveillance intervals.”
Advertisement
Risk-stratification is an active area of BE research, with opportunities to further refine the criteria, Dr. Thota says. “The more we can study the tissue pathology of BE patients, the more accurately we can identify and fine-tune their risk of progression,” she says. “Then we can focus our resources on patients with the highest risk of progression.”
To further improve accuracy, future studies will target larger patient populations and include additional medical centers.
Image: TissueCypher detected multiple high-risk features in this nondysplastic biopsy from a patient with short-segment BE and no visible lesions who progressed to high-grade dysplasia 2.5 years later. TissueCypher scored this case 6.4/high risk for progression to HGD/EAC within 5 years. © 2019 Cernostics. Re-used with permission.
Advertisement
Advertisement

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units

Addressing rare disease and challenging treatment course in an active young patient