Surgeons reassured by low risk of harm
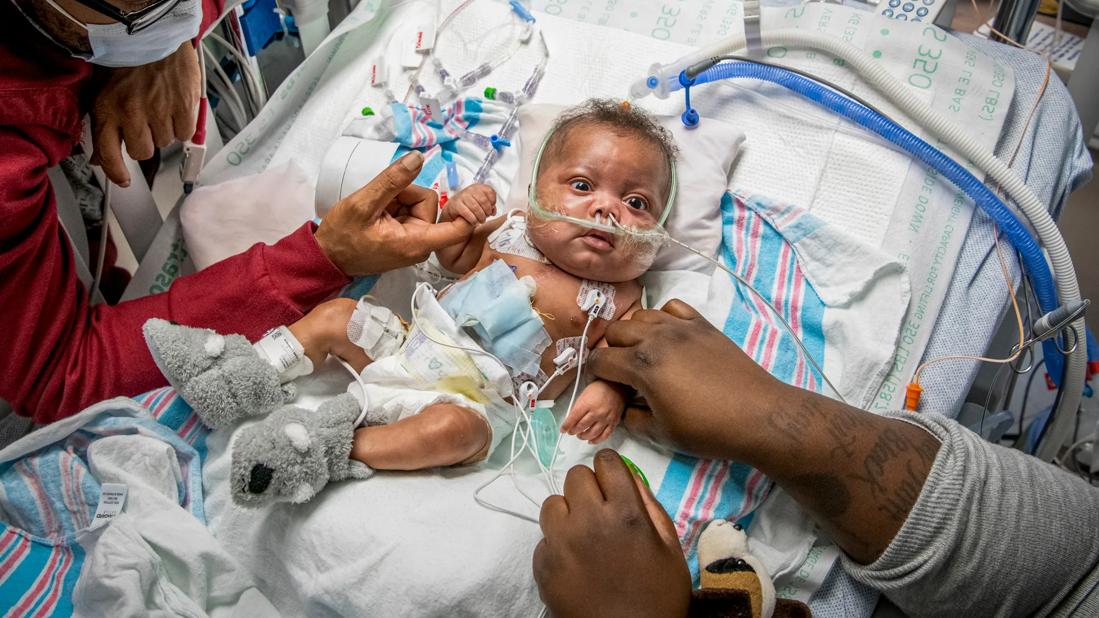
Use of steroids in infants undergoing heart surgery with cardiopulmonary bypass is a routine practice designed to reduce inflammation and improve the likelihood of a smooth recovery. Whether it causes harm has been debated. Now, the largest randomized, prospective, controlled clinical trial ever conducted in pediatric cardiac surgery has put the issue to rest.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The answer is no,” says Tara Karamlou, MD, a pediatric and congenital heart surgeon at Cleveland Clinic Children’s who participated in the Steroids to Reduce Systemic Inflammation after Infant Heart Surgery (STRESS) trial. “I think it demonstrated that centers can use steroids in these highly complex patient populations without fear of deleterious things happening down the road.”
STRESS was a prospective, placebo-controlled, registry-based trial that randomized 1200 of 1263 infants less than 1 year of age undergoing heart surgery with cardiopulmonary bypass at 24 sites to receive prophylactic methylprednisone (30 mg/kg of body weight) or placebo in the pump-priming fluid. Results were published in the New England Journal of Medicine on Nov. 6, 2022.
The primary endpoint was a composite of death, heart transplantation or any of 13 major complications. The likelihood of a worse outcome did not differ significantly between the steroid group and the placebo group. However, need for postoperative insulin for hyperglycemia was greater in the steroid group (19% vs. 6.7%).
The unadjusted secondary analyses showed an odds ratio for a worse outcome to be 0.82 and a win ratio of 1.15 in the methylprednisone group, compared with the placebo group. These findings demonstrate a benefit with steroids that Dr. Karamlou says should not be discounted.
“I feel there is probably an incremental advantage to using steroids in populations that are too small to study as individual subsets,” she says. “Some covariables may be obscuring the benefit. It’s an aggregate population, so in a large trial like this you may lose the ability to discern benefit in key populations.”
Advertisement
Dr. Karamlou was not deterred by the slight increase in risk of hyperglycemia, as it may be resolved with a small amount of insulin.
The main message was that no adverse effects from methylprednisone were seen. This is important to centers like Cleveland Clinic that use methylprednisone in all babies, including neonates, requiring cardiopulmonary bypass.
“Steroids have a lot of systemic effects, but they are the best tool we have to avoid the deleterious effects of cardiopulmonary bypass in small babies. Steroids may improve the perioperative convalescence of patients following open heart surgery because they often expedite the ability to get patients extubated, have their sternum closed and leave the ICU,” she says.
While most centers follow the STRESS trial protocol and give a single dose of steroids in the pump prime, Cleveland Clinic normally gives a dose the evening before surgery and another dose in the pump prime.
“Some centers give three doses, adding the third eight to 10 hours after surgery. We don’t do the post-surgical dose, but we will continue giving our neonatal patients steroids in the pump prime and also the evening before surgery,” says Dr. Karamlou.
A tangential benefit of STRESS was its successful construct as a trial within a registry. Participants in all 24 sites were enrolled in the Society of Thoracic Surgeons Congenital Heart Surgery Database. This reduced the costs of the study from an estimated $10 million to $3.2 million.
Dr. Karamlou calls the concept and execution of the study “transformative.”
Advertisement
“Pediatric heart surgery is a resource-limited enterprise. Most pediatric cardiac surgery is a money-loser considering 90% of reimbursement in most states comes from Medicaid,” she says.
“Executing a randomized trial using registry data, and having it validated within the context of a National Institutes of Health-funded prospective, placebo-controlled trial has never been done before.
“The ability to conduct this type of trial at a reduced cost is critical for pushing the needle on our specialty forward,” she says.
Advertisement
Advertisement

Medical and surgical perspectives on current and emerging uses of ECMO and Impella

Least-invasive open-heart AVR option to date yielded rapid recovery in all cases

Launch of the tool promises to reshape quality assessment across the specialty
Lead dwell time and manufacturer emerge as independent predictors of success in registry study
The case for a thoughtful approach to CTO and minimally invasive options for CABG

How the tool could help physicians alter management in real time
Preoperative Impella 5.5 placement can provide a critical safety net for high-risk patients
Technique may lay groundwork for personalized decision-making in procedural intervention