Study matches momelotinib against danazol
After 48 weeks of treatment, patients with symptomatic myelofibrosis who were previously treated with another JAK inhibitor were more likely to have symptoms managed with momelotinib than with danzol, according to recent phase 3 study results, which were published in The Lancet.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The MOMENTUM study found after 24 weeks of treatment, 25% of patients experienced improvement in overall symptoms; 31% experienced improvement in anemia; with no need for a blood transfusion and 23% had reduced spleen size. 48 weeks into the study, the researchers again assessed patients to determine how well tolerated the medication was as well as how effective it was at maintaining initial benefits, including in those patients with low platelet counts.
Momelotinib promises to be a two for one,” says Aaron T. Gerds, MD, MS, study co-author and Deputy Director for Clinical Research at the Taussig Cancer Institute. “Not only do we see spleen size and symptom burden improvements as we would expect with any JAK inhibitor, but we also see anemia benefits as well.” Symptom management, including anemia and spleen benefits, was maintained after 48 weeks. Additionally, the overall survival benefits and chances of avoiding acute myeloid leukemia were sustained.
The medication continued to be well tolerated by most patients. The most common side effects were weight loss, diarrhea, high blood pressure and fever. Additionally, some patients experienced low platelets, low red blood cells or low white blood cells. Notably, the side effects did not appear to worsen after a longer period taking momelotinib.
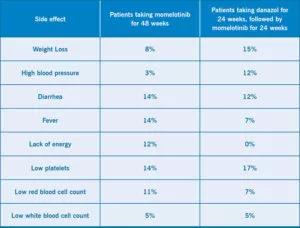
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a37a0d40-40a6-42f0-9807-85c22776c5dd/22-CNR-3344410-CQD-Chart-Inset-800x608-1-300x228_jpg)
Dysregulated JAK-STAT signaling in myelofibrosis drives overproduction of inflammatory cytokines, bone marrow fibrosis as well as clonal proliferation, resulting in extramedullary hematopoiesis and spleen enlargement. Chronic inflammation is a hallmark of the disease, resulting in anemia, elevated hepcidin, dysregulated iron metabolism and hyperactivation of ACVR1, a protein that regulates iron in the blood. While JAK inhibitors are often used to treat the disease, those that are currently FDA approved do not actively address anemia. By blocking ACVR1, researchers found that momelotinib could ameliorate anemia, which is a negative prognostic indicator in the disease.
Advertisement
185 patients previously treated with another JAK inhibitor participated in the study. Most study participants had previously experienced numerous side effects. Nearly half of study participants were already anemic and needed numerous blood transfusions prior to joining the study. For the first 24 weeks of the study, 130 participants received 200mg of the JAK inhibitor momelotinib daily while 65 participants received 300 mg of the anabolic steroid danazol daily. After 24 weeks, all the study participants were treated with 200 mg of momelotinib.
In terms of symptom management, momelotinib was more effective than danazol for all measures.
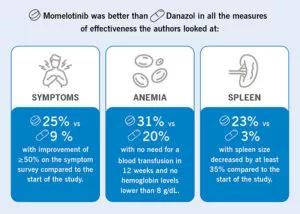
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/da97e43d-b075-4191-b4a2-a00942afd29f/22-CNR-3344410-Inset2-Comparison-800x570-1-300x214_jpg)
The anemia analysis was non-inferiority. The statistical test for that endpoint used a one-sided p-value.
Patients who experienced improved symptoms after the first 24 weeks sustained those improvements after 48 weeks. Patients who were transfusion-independent at 24 weeks remained so at 48 weeks. The average hemoglobin levels improved or stayed the same over time for the majority of those taking momelotinib. Additionally, those who had a smaller spleen side after 24 weeks continued to do so after 48 weeks.
There was a trend toward better overall survival with the momelotinib arm at the 28-week analysis. With the subsequent crossover the survival curve of the patients on the danzol arm overlapped with that of the momelotinib arm.
Learn more in our recent podcast.
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors