Key learnings from DESTINY trials
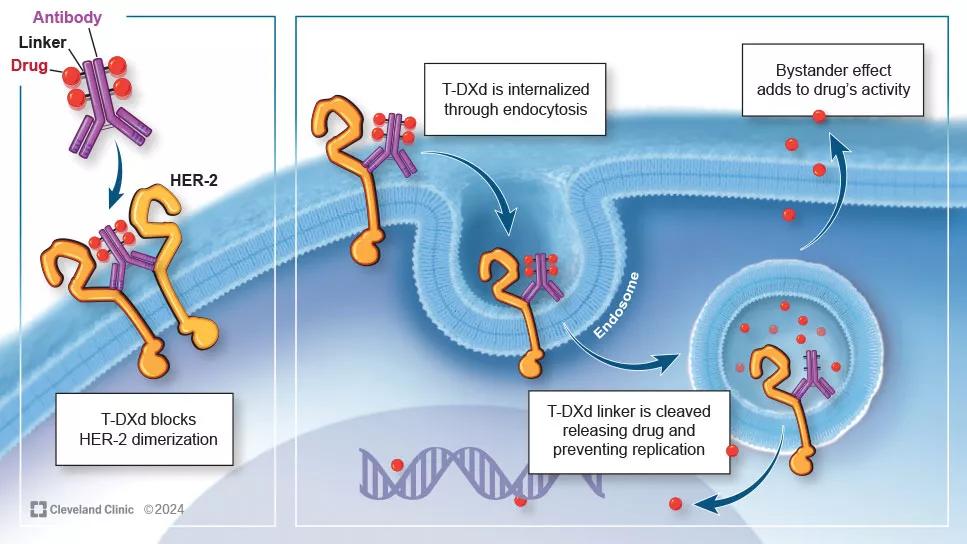
Antibody drug conjugates like trastuzumab deruxtecan (T-DXd) are generating great interest for the treatment of both HER2-positive and HER2-low breast cancer. Cleveland Clinic medical oncologist Azka Ali, MD, recently coauthored a paper in Current Oncology Reports highlighting the most notable research findings to date for this medication as well as optimal management practices to employ when prescribing it.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
T-DXd consists of an antibody that targets HER2 and then releases the cytotoxin into the desired location. Like other ADCs, what makes T-DXd interesting is that there is less off-target toxicity due to its active mechanism. What makes it special is that it does exert notable bystander effect, which is thought to add to the drug’s activity.
The medication was initially approved for treatment of patients with HER2-positive disease, but recently researchers learned that it also had significant activity against a group of patients previously classified as HER2-negative (HER2 immunohistochemistry [IHC] 1+ or 2+ with a negative in situ hybridization) that are now reclassified as “HER2-low”.
“With the expansion in the patient eligibility from the above criteria, it means now there are some hormone receptor (HR)-positive and triple negative patients that would be eligible for a new line of therapy, and that’s very exciting,” says Dr. Ali. Currently this medication is also standard of care as second-line therapy for HER2-positive patients after relapse with chemotherapy, trastuzumab and pertuzumab.
There is some early data that T-DXd provides activity for HER2-negative (i.e., HER2 IHC 0) patients, and there are trials under way to study this benefit. A national trial (DESTINY-Breast06) is also underway to determine if T-DXd is effective after progression on endocrine therapy for HR+/HER2-low and HER2-negative (IHC 0) patients.
A distinctive feature of the medication is its ability to penetrate the central nervous system (CNS). Many cancer therapies cannot traverse the blood-brain barrier. This is a concern when treating patients with HER2-positive breast cancer since they are at high risk of brain metastasis. Researchers believe that T-DXd has notable intracranial activity.
Advertisement
The growth of this type of targeted therapy means all patients with breast cancer should have immunohistochemistry and FISH testing performed at diagnosis. This will help identify HER2 status, which is necessary to identify the subset of patients that may be HER2-low, previously categorized as HER2-negative This data may prove useful as new therapies become available for targeting additional biomarkers.
The toxicity profile of ADCs like T-DXd differ from chemotherapy. The notable adverse events to watch for are:
Interstitial lung disease (ILD). “Although most incidents of ILD in DESTINY trials were grade one or two, there were some grade 3 or higher cases and there were deaths related to ILD on the trials, so this needs to be taken seriously,” cautions Dr. Ali.
To monitor for ILD, the recommendation is to perform high-resolution CT scans at baseline and every six to twelve weeks. If the ILD is asymptomatic, the recommendation is to hold the drug, treat with steroids and then resume the drug if ILD resolves in <28 days.
If the ILD is symptomatic (ex: shortness of breath), a scan is recommended. If the scan reveals ILD, the medication needs to be permanently discontinued. “It can be hard to know the cause of ILD," says Dr. Ali. "In these cases, we consult with our pulmonology colleagues to rule out alternative causes but in clinical practice, that can often be very challenging.”
Cardiac function. Performing echocardiograms every three to six months is recommended for patients on T-DXd, since it can cause cardiac function decline. Rates of this side effect are low (grade 3 or lower in 0 to 1.5% of patients across DESTINY trials), and occur more frequently in heavily pretreated patients.
Advertisement
Nausea. T-DXd is considered a high emetogenic medication. As such, physicians should preemptively and aggressively treat for nausea. The current recommendation is to prescribe the combination of olanzapine, dexamethasone, an NK-1 inhibitor and a selective 5-HT3 receptor antagonist.
“As we learn more about these novel drugs, we should be careful in identifying toxicities and have a low threshold for dose reduction if we reached a toxicity that is more than what was encountered in clinical trials,” says Dr. Ali. “This is where the package insert is very helpful. It contains a wealth of information to tell us exactly how trials were performed and what the drug manufacturer recommends for dose reductions.”
Although T-DXd holds much promise, researchers are seeking more data to better understand its ideal application. "One hot question that continues to vex us is how do we sequence some of these novel therapies?" says Dr. Ali. "Sacituzumab govitecan is another ADC that has clinical activity in both HR-negative and HR-positive patients, and we do not know if that should be used before or after T-DXd. Both options are now available in the second line setting but we don’t yet have enough data to know which should come first.”
Another area of interest is which medication is best equipped to protect against brain metastasis. Although there are data to support that T-DXd has a remarkable ability to traverse the blood-brain barrier, this was only demonstrated in a small number of patients (double digits). The three-drug regimen Herceptin, tucatinib and capecitabine also demonstrated CNS penetration in a phase 3 study of hundreds of patients. Until there is more robust data available about T-DXd in this setting, the combination of herceptin, tucatinib and capecitabine remains the preferred option for many.
Advertisement
The knowledge base we have about T-DXd is growing. “Therapies like this are enabling treatment to become more personalized,” says Dr. Ali. “As T-DXd moves up in earlier in the line of treatment, we continue to seek answers to questions about resistance mechanisms, manageable safety profiles and optimal sequencing.”
Advertisement
Advertisement

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Reconsidering axillary lymph node dissection as well as depth of surgical margins

Ultra-Hypofractionated Whole Breast Irradiation and Partial Breast Irradiation Reduce Many Toxicities

Best practices for reducing toxicities

Partnerships with local social service agencies key to program success

Ongoing clinical validation refine breast cancer risk substratification

Phase 3 trial found no survival differences between weekly or biweekly doxorubicin/cyclophosphamide or between weekly or biweekly paclitaxel

Findings strengthen evidence for risk-reducing procedures