Tag debug info: client: {"assets":{},"datasets":{},"live":{},"projects":{},"users":{},"observable":{"assets":{},"datasets":{},"live":{},"projects":{},"users":{}}} Now: 1770472568237 Cache Key: cqdTagPageBySlug:cardiac-implantable-electronic-device-cied fetchCache[cqdTagPageBySlug:cardiac-implantable-electronic-device-cied].expirationTime: falsey fetchCache[cqdTagPageBySlug:cardiac-implantable-electronic-device-cied]. seconds remaining: falsey All fetchCache expiration times: -- Key: cqdNotFoundPage, seconds remaining: -369 -- Key: cqdTagPageBySlug:pediatric-liver-transplant, seconds remaining: -369 -- Key: cqdPostsByTag:cqd-migrated-tag-22633,1,10, seconds remaining: -299 -- Key: cqdTagPageBySlug:renal-artery-stenosis, seconds remaining: 3691 -- Key: cqdPostsByTag:cqd-migrated-tag-21377,1,10, seconds remaining: 3763 -- Key: cqdTagPageBySlug:ultra-widefield-angiography, seconds remaining: 4560 -- Key: cqdPostsByTag:cqd-migrated-tag-22860,1,10, seconds remaining: 4729 -- Key: cqdTagPageBySlug:sars-cov-2, seconds remaining: 5910 -- Key: cqdPostsByTag:cqd-migrated-tag-21907,1,10, seconds remaining: 5943 -- Key: cqdTagPageBySlug:bronchial-thermoplasty-bt, seconds remaining: 6848 -- Key: cqdPostsByTag:cqd-migrated-tag-1703,1,10, seconds remaining: 6910 -- Key: cqdTagPageBySlug:end-stage-renal-disease-esrd, seconds remaining: 7727 -- Key: cqdPostsByTag:cqd-migrated-tag-1038,1,10, seconds remaining: 7805 -- Key: cqdTagPageBySlug:heart-failure, seconds remaining: 8623 -- Key: cqdPostsByTag:cqd-migrated-tag-74,3,10, seconds remaining: 8711 conditions: -- false, -- NA, -- NA, -- NA -- false Cache miss for key cqdTagPageBySlug:cardiac-implantable-electronic-device-cied - retrieving from Sanity CCCache.dataFetchCount: 6075 Cache cleanup seconds remaining: 27727
Advertisement
Advertisement
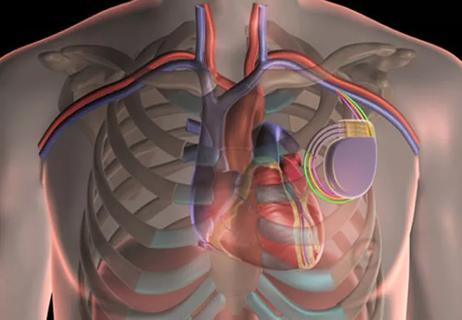
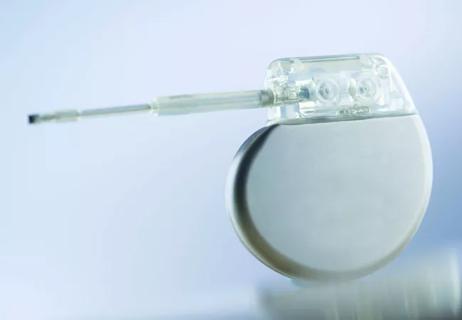
Tag: cardiac implantable electronic device (CIED)
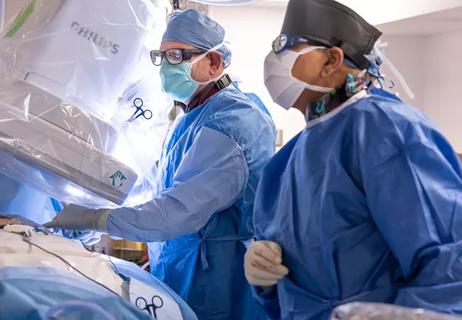
First-in-world implantation performed at Cleveland Clinic
Results achieved despite increased complexity and venous occlusive disease
Results also show eagerness to learn more
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Rendered: Sat Feb 07 2026 13:56:08 GMT+0000 (Coordinated Universal Time)
9500 Euclid Avenue, Cleveland, Ohio 44195 |
800.223.2273 | ©
2026 Cleveland Clinic. All Rights Reserved.