Tag debug info: client: {"assets":{},"datasets":{},"live":{},"projects":{},"users":{},"observable":{"assets":{},"datasets":{},"live":{},"projects":{},"users":{}}} Now: 1770470143900 Cache Key: cqdTagPageBySlug:myelofibrosis fetchCache[cqdTagPageBySlug:myelofibrosis].expirationTime: falsey fetchCache[cqdTagPageBySlug:myelofibrosis]. seconds remaining: falsey All fetchCache expiration times: -- Key: cqdNotFoundPage, seconds remaining: 8514 -- Key: cqdTagPageBySlug:kidney-injuries, seconds remaining: -2625 -- Key: cqdPostsByTag:cqd-migrated-tag-18242,1,10, seconds remaining: -2549 -- Key: cqdTagPageBySlug:functional-neurological-disorder, seconds remaining: -1516 -- Key: cqdPostsByTag:cqd-migrated-tag-25628,1,10, seconds remaining: -1453 -- Key: cqdTagPageBySlug:pablo-recinos, seconds remaining: -1073 -- Key: cqdPostsByTag:cqd-migrated-tag-1731,1,10, seconds remaining: -993 -- Key: cqdTagPageBySlug:transcatheter-asd, seconds remaining: -442 -- Key: cqdPostsByTag:cqd-migrated-tag-17297,1,10, seconds remaining: -373 -- Key: cqdTagPageBySlug:pneumonia-risk, seconds remaining: -158 -- Key: cqdPostsByTag:cqd-migrated-tag-1797,1,10, seconds remaining: -88 -- Key: cqdTagPageBySlug:cytoreductive-surgery, seconds remaining: 472 -- Key: cqdPostsByTag:cqd-migrated-tag-18563,1,10, seconds remaining: 544 -- Key: cqdTagPageBySlug:serum-chloride, seconds remaining: 3025 -- Key: cqdPostsByTag:cqd-migrated-tag-20061,1,10, seconds remaining: 3097 -- Key: cqdTagPageBySlug:achalasia, seconds remaining: 3807 -- Key: cqdPostsByTag:cqd-migrated-tag-4235,1,10, seconds remaining: 3882 -- Key: cqdTagPageBySlug:mariela-herrera, seconds remaining: 4476 -- Key: cqdPostsByTag:cqd-migrated-tag-2093,1,10, seconds remaining: 4548 -- Key: cqdTagPageBySlug:affordable-care, seconds remaining: 5019 -- Key: cqdPostsByTag:cqd-migrated-tag-1490,1,10, seconds remaining: 5094 -- Key: cqdTagPageBySlug:lisa-grove, seconds remaining: 5290 -- Key: cqdPostsByTag:cqd-migrated-tag-24377,1,10, seconds remaining: 5360 -- Key: cqdTagPageBySlug:hvla-manipulations, seconds remaining: 5534 -- Key: cqdPostsByTag:cqd-migrated-tag-24225,1,10, seconds remaining: 5594 -- Key: cqdTagPageBySlug:sex-based-differences, seconds remaining: 5965 -- Key: cqdTagPageBySlug:vickram-tejwani, seconds remaining: 6072 -- Key: cqdPostsByTag:cqd-migrated-tag-23470,1,10, seconds remaining: 6039 -- Key: cqdPostsByTag:cqd-migrated-tag-26854,1,10, seconds remaining: 6144 -- Key: cqdTagPageBySlug:organ-supply, seconds remaining: 6232 -- Key: cqdPostsByTag:cqd-migrated-tag-26640,1,10, seconds remaining: 6298 -- Key: cqdTagPageBySlug:stent-grafting, seconds remaining: 6685 -- Key: cqdPostsByTag:cqd-migrated-tag-4170,1,10, seconds remaining: 6758 -- Key: cqdTagPageBySlug:james-fernandez, seconds remaining: 8514 -- Key: cqdPostsByTag:cqd-migrated-tag-879,1,10, seconds remaining: 8596 -- Key: cqdTagPageBySlug:osteogenesis-imperfecta-oi, seconds remaining: 9727 -- Key: cqdPostsByTag:cqd-migrated-tag-3473,1,10, seconds remaining: 9795 conditions: -- false, -- NA, -- NA, -- NA -- false Cache miss for key cqdTagPageBySlug:myelofibrosis - retrieving from Sanity CCCache.dataFetchCount: 4783 Cache cleanup seconds remaining: 25965
Advertisement
Advertisement
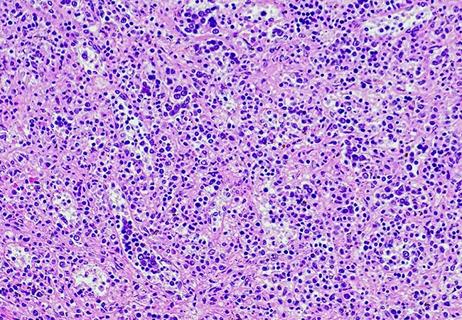
Phase 1 study found mutant calreticulin-specific monoclonal antibody brings promising results with no dose-limiting toxicities
Combination therapy doubles the number of meaningful spleen volume responses over monotherapy
Approach resulted in transfusion independence and durable anemia response
Study matches momelotinib against danazol
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Momelotinib, a new JAK inhibitor, inches closer to market approval
Dose-finding trial adds to evidence of efficacy
Interim results of phase 2 trial are promising
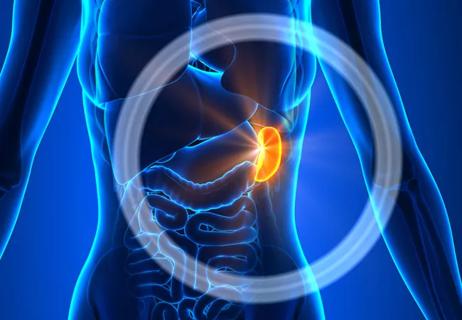
Second (or third) time’s a charm in reducing splenomegaly and other symptoms
Final results of PERSIST-2 confirm benefit; PAC203 enrolling
Rendered: Sat Feb 07 2026 13:15:44 GMT+0000 (Coordinated Universal Time)
9500 Euclid Avenue, Cleveland, Ohio 44195 |
800.223.2273 | ©
2026 Cleveland Clinic. All Rights Reserved.