Failure to screen can be deadly
Cleveland Clinic doctors decided to perform a retrospective study after a patient who was treated with the drug rituximab died from reactivated hepatitis B (HB) to determine the scope of the problem and how well physicians were adapting to changing guideline recommendations.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“This patient was diagnosed with cancer and given rituximab. Later he developed reactivation HB because of the dormant hepatitis B in his liver cells,” says Mahnur Haider, MD, a third-year internal medicine resident, who led the study and presented it today at the American Association for the Study of Liver Diseases The Liver Meeting 2018.
“Guidelines for screening and management of those at risk have changed significantly in recent years,” adds hepatologist William Carey, MD, who is also an author on the study. “Any patient with HB core antibody (anti-HBc), regardless of HB surface antigen (HBsAg) status, is at risk for reactivation hepatitis B. Thus, pre-treatment screening must include testing for this antibody.”
The researchers say a literature search uncovered a recent meta analysis of 55 studies looking at HB screening and physician compliance that showed that there was about an 8.3 percent incidence of HB reactivation in that context. With that in mind, Drs. Haider and Carey and their colleagues decided to conduct a retrospective study looking at Cleveland Clinic patients who received rituximab between 2005-2017.
For their investigation, the researchers defined complete screening as testing for HB surface antigen and core antibody prior to treatment with rituximab. Patients with HB core antibody with or without HB surface antigen were considered at-risk of HB reactivation. They also looked at how often at-risk patients were given prophylactic nucleoside analogs (NA) — which help prevent viral replication and can stop an HB reactivation.
Advertisement
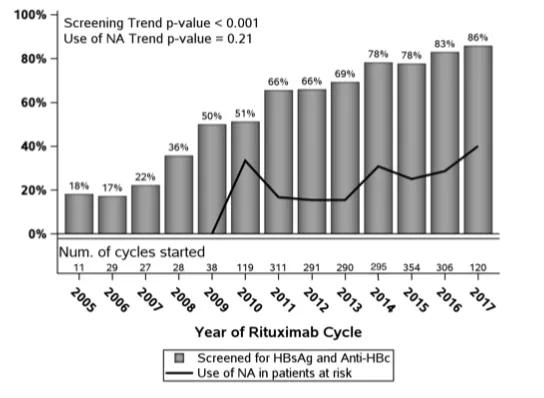
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/832e6149-2756-4de6-af02-38a6694bf643/bargraph_png)
Overall, they found that among the 2,219 patients who received rituximab,1,917 (86.4 percent) are currently screened. There has been a nearly fourfold increase in the screening rate over the past 10 years.
The good news is the very low rate of hepatitis B reactivation. Only 8 cases of HB reactivation were found, a remarkably low 0.4 percent. Part of the explanation for the low incidence is that only a small proportion (5.3 percent) of those tested had previous exposure to hepatitis B (defined as a positive anti-HBc antibody). The total incidence of reactivation was 8 percent, three in those completely screened versus five who were not screened prior to treatment with rituximab. The reactivated patients tested negative for HB surface antigen at the time of screening.
The bad news is the continued very high mortality rate (25 percent) in those affected by reactivation. Hepatitis B drugs given before rituximab appeared to be totally effective. No case of reactivation hepatitis B was seen in the 19 at-risk patients who were given NA therapy as prophylaxis prior to administration of rituximab. However, when NA was started after rituximab one (of 12) at-risk patient reactivated.
“When you look at the data, you can see that screening has gone up from about 20 percent of patients in 2005 to about 86 percent in 2017,” says Dr. Haider. “But there’s still room for improvement, especially in terms of giving antiviral prophylaxes prior to treatment with rituximab. If a patient reactivates the fatality rate is high but if you administer the prophylaxis, the chance of reactivation is very low.”
Advertisement
Both Drs. Haider and Carey say the data indicate that more physicians need a better understanding what blood test results identify those at risk for HB reactivation.
Dr Carey says too often physicians think that if there’s no surface antigen in a patient’s blood then that patient is not at risk of reactivation. But he notes the virus can still lurk in the nucleus of liver cells, so it’s important to also screen for HB core antibody. “We found people who were surface antigen positive and reactivated,” he explains, “and people who were surface antigen negative and reactivated.”
Dr Haider says they hope to collaborate with Cleveland Clinic Pharmacy to create an alert system within the electronic medical record for patients for whom rituximab has been prescribed. Such an alert would have three features: 1) identification of those whose anti-HBc status is known; 2) recommendation for anti HBc testing for those who have not had it; 3) prompts to the healthcare provider to consider prescribing NA prior to rituximab for all who test positive for anti HBc.
Advertisement
Advertisement

Benefits of neoadjuvant immunotherapy reflect emerging standard of care

Multidisciplinary framework ensures safe weight loss, prevents sarcopenia and enhances adherence

Study reveals key differences between antibiotics, but treatment decisions should still consider patient factors

Key points highlight the critical role of surveillance, as well as opportunities for further advancement in genetic counseling

Potentially cost-effective addition to standard GERD management in post-transplant patients

Findings could help clinicians make more informed decisions about medication recommendations

Insights from Dr. de Buck on his background, colorectal surgery and the future of IBD care

Retrospective analysis looks at data from more than 5000 patients across 40 years