Case highlights potential benefits of this staged, dual-therapy approach
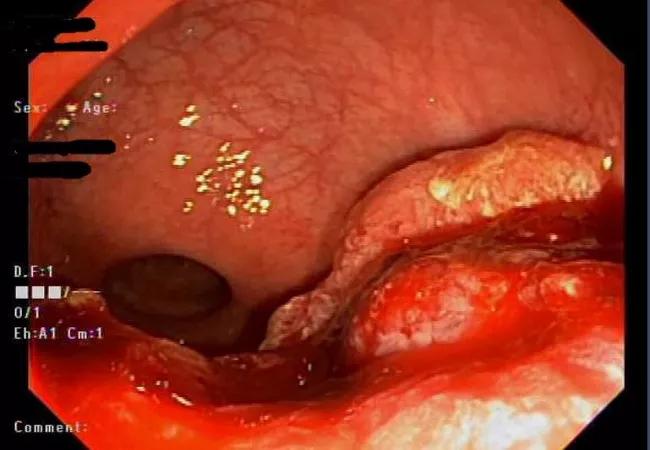
A 41-year-old woman who had experienced four months of rectal bleeding was referred to Cleveland Clinic and Emre Gorgun, MD, Section Chief of Colorectal Surgical Oncology, after a diagnostic colonoscopy identified a potentially malignant rectal mass. Flexible endoscopy inspection and biopsy confirmed a diagnosis of rectal adenocarcinoma. Clinical staging classified the cancer as stage IIA (T3N0).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
After the patient completed chemoradiation in May 2020, a restaging MRI scan revealed a moderate response. Cleveland Clinic’s Colorectal Cancer Multidisciplinary Tumor Board recommended that the patient undergo total neoadjuvant therapy (TNT).
Historically, chemoradiation followed by total mesorectal excision and adjuvant chemotherapy has been the standard of care for locally advanced rectal cancer.
TNT is a relatively new organ-sparing and distant micrometastases-prevention protocol from the Organ Preservation in Rectal Adenocarcinoma trial in which patients diagnosed with stage II or III locally advanced rectal cancer receive upfront chemoradiotherapy and consolidation chemotherapy. Patients who achieve a complete response on multimodal assessment following TNT are placed on active surveillance, which involves physical exam, flexible sigmoidoscopy, monitoring of carcinoembryonic antigen levels and MRI and CAT scans at regular intervals for at least five years. About 30 percent of patients can avoid surgery with this approach.
The patient completed TNT chemotherapy in November 2020 and showed a complete response. The following month, she entered the watch-and-wait protocol.
During a subsequent surveillance flexible scope evaluation, clinicians detected polyp regrowth. Pathology revealed a rectal scar and some residual adenoma with low-grade dysplasia.
The patient underwent endoscopic submucosal dissection (ESD) of her rectal scar lesion in October 2021. Recent advances in equipment and techniques have made it possible to endoscopically remove precancerous, cancerous and complex polypoid lesions that traditionally required surgical resection, using ESD or other endoluminal approaches.
Advertisement
Pathology following ESD showed the patient’s dissection margins were negative for dysplasia. The tumor board recommended that she continue on the watch-and-wait protocol.
Her most recent follow-up scans confirmed no evidence of cancer recurrence.
“She remains disease-free and the site that we treated with advanced endoscopy is perfectly healed, with nice epithelialization,” says Dr. Gorgun, who is also Director for Lower GI of Cleveland Clinic’s Endoluminal Surgery Center.
Were it not for this multimodal TNT/ESD option, the patient might have required major surgery to remove her rectum, leaving her with a permanent ostomy, Dr. Gorgun explains. This combined approach “gave us the extra comfort that we can monitor this patient further without her undergoing a major operation.”
Endoscopy at the time of diagnosis shows the rectal adenocarcinoma.
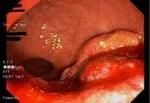
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/55f7908d-9ecb-4c5c-9171-aa95a5d2d809/22-DDI-2684108-ESD-after-TNT-DIAGNOSIS-800x550-1-150x103_jpg)
After total neoadjuvant therapy.
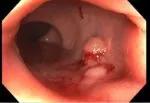
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f0dca219-6007-4ab2-a17e-665a85b9fdd7/22-DDI-2684108-ESD-after-TNT-AFTER-TNT-800x550-1-150x103_jpg)
After endoscopic submucosal dissection.
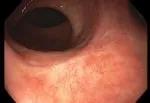
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4663ad80-94c2-42bb-addb-6ce1375ca53d/22-DDI-2684108-ESD-after-TNT-AFTER-ESD-800x550-1-150x103_jpg)
Pathology following TNT and ESD.
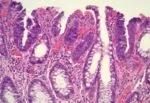
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3885e520-5a79-4c59-aeaf-fb95ae71a283/Pathology-image-of-last-ESD-after-TNT-1-800x550-1-150x103_jpg)
Combining TNT with ESD is relatively new and the option is not widely available. Cleveland Clinic was one of the first centers in the United States to incorporate TNT into standardized care paths and to pioneer the dual TNT/ESD strategy. Its use is not based on an individual surgeon’s decision, but rather on expert consensus. The tumor board discusses evidence-based information to support an individualized treatment plan for each patient.
Cleveland Clinic offers TNT to clinically appropriate colorectal cancer patients. Treatment takes approximately six months, and up to 50% of patients have a complete response.
Advertisement
For patients with evidence of residual cancer, abnormal tissue or high-grade dysplasia after TNT, ESD is a potential next step to remove remaining complex polyps or lesions detected during surveillance. Adding ESD to a patient’s treatment plan maintains an organ-sparing strategy.
ESD has been used for other cancer patients, such as those with early esophageal and gastric cancers. Dr. Gorgun and colleagues adapted the technique to colorectal cancer management.
Although difficult to directly compare with traditional major surgery outcomes, in general TNT and ESD patients have minimal side effects or complications, he says. “So it’s certainly much more beneficial.”
“This is a cutting-edge, innovative, and futuristic approach,” Dr. Gorgun says.
He and his colleagues plan to explore ways to optimize the safety and effectiveness of this combination treatment strategy going forward.
Advertisement
Advertisement

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units