New study sheds light on how long you can safely wait
In general, delay in definitive management after diagnosis of malignancies impacts progression or metastases rates. However, given the slow growing nature of prostate cancer (PCa), treatment delay may not have a significant impact on recurrence.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In this study, researchers evaluated the impact of treatment delay on the biochemical recurrence (BCR) rate of PCa, specifically in patients with intermediate- to high-risk pathology.
The study was presented in a poster session at the American Society of Clinical Oncology’s 2017 Genitourinary Cancers Symposium by Sudhir Isharwal, MD, clinical fellow at Cleveland Clinic Glickman Urological & Kidney Institute.
The retrospective review of Cleveland Clinic’s institutional database included patients who underwent radical prostatectomy (RP) between 2004 and 2014. Patients were included if they had data for initial PSA, clinical stage and Gleason score. A well-established and validated preoperative nomogram was used to predict the five-year biochemical recurrence probability. Using a multivariable model, researchers evaluated the actual risk of BCR (PSA>0.2) stratified by time from diagnosis to treatment, controlling for age and using the preoperative nomogram. The same analysis was conducted in the intermediate or high-risk category cohort of the National Comprehensive Cancer Network (NCCN).
A total of 3,029 patients met inclusion criteria. Median age for the entire cohort was 60 years. Median time from diagnosis to treatment was 77 days (IQR 54-110). Median overall followup time was 25 months. Group 1 (1,871 patients, 61.7 percent) experienced a delay of less than 90 days. Group 2 (955 patients, 31.5 percent) experienced delays of 90-180 days, and Group 3 (203, 6.7 percent) experienced delays of >180 days.
Advertisement
BCR occurred in 9.6 percent, 9.6 percent and 4.9 percent of patients in Groups 1, 2 and 3 respectively (p=0.08). Out of the entire cohort, 1,837 patients (60.6 percent) had NCCN intermediate- or high-risk disease. On Cox multivariable analysis, there was no significant difference in risk of BCR stratified by time to radical prostatectomy in the entire cohort or in patients with intermediate- or high-risk disease (see Table 1).
The majority of patients in this cohort underwent surgery within 180 days of diagnosis. Even among those classified as NCCN intermediate- or high-risk, the delay in treatment did not have significant effect on the risk of BCR.
“While patients with high-risk pathology should without doubt receive prompt treatment, our data suggest that patients and urologists have time to obtain genomic or imaging information and seek second opinions or referral for surgery without significantly affecting the BCR,” Dr. Isharwal says.
“Diagnosis of prostate cancer causes a great deal of anxiety in patients and they want to get treatment as soon as possible,” he continues. “Although there shouldn’t be any undue delay in treatment, findings from our study show that waiting even up to six months is safe. This time period provides surgeons the ability to get additional testing such as MRI and genomic assessments. Also, patients can feel comfortable seeking out a second opinion or traveling to a center of excellence for surgery.”
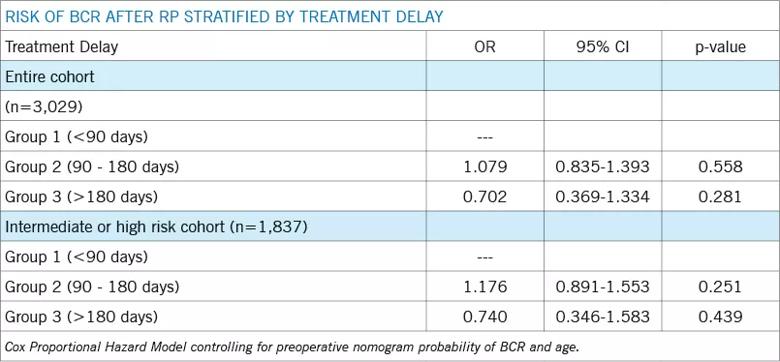
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/bd5f4501-6fa1-4f73-a819-b63d5ae84f8c/Chart-Isharwal_jpg)
Table 1
Advertisement
Advertisement

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units

Addressing rare disease and challenging treatment course in an active young patient