Device provides alternative to conservative management and surgical procedures
More than one in 10 adult women in a population-based study indicated they have fecal incontinence, with one in 15 experiencing moderate to severe symptoms. Yet the condition often goes untreated.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“It’s a bigger problem than most people realize,” says Shannon Wallace, MD, a physician in the Division of Urogynecology and Pelvic Floor Disorders at the Cleveland Clinic Women’s Institute. “Not many primary care physicians or gynecologists really ask about fecal incontinence, and patients don’t bring it up because it’s embarrassing and uncomfortable.”
Until just a few years ago, patients who broached the topic with physicians had two primary treatment paths – conservative management or surgical procedures. In 2015, Pelvalon introduced the Eclipse™ System, the first vaginal insert designed to provide bowel control. (Laborie Medical Technologies Corp. acquired Pelvalon in October 2021.)
“Patients didn’t have many options between doing pelvic floor physical therapy and making dietary changes or ending up with some surgical procedure. The benefit of the Eclipse is that it targets the middle market,” says Dr. Wallace, who fitted more than a dozen patients with the device during her Female Pelvic Medicine and Reconstructive Surgery Fellowship at Stanford University Hospital.
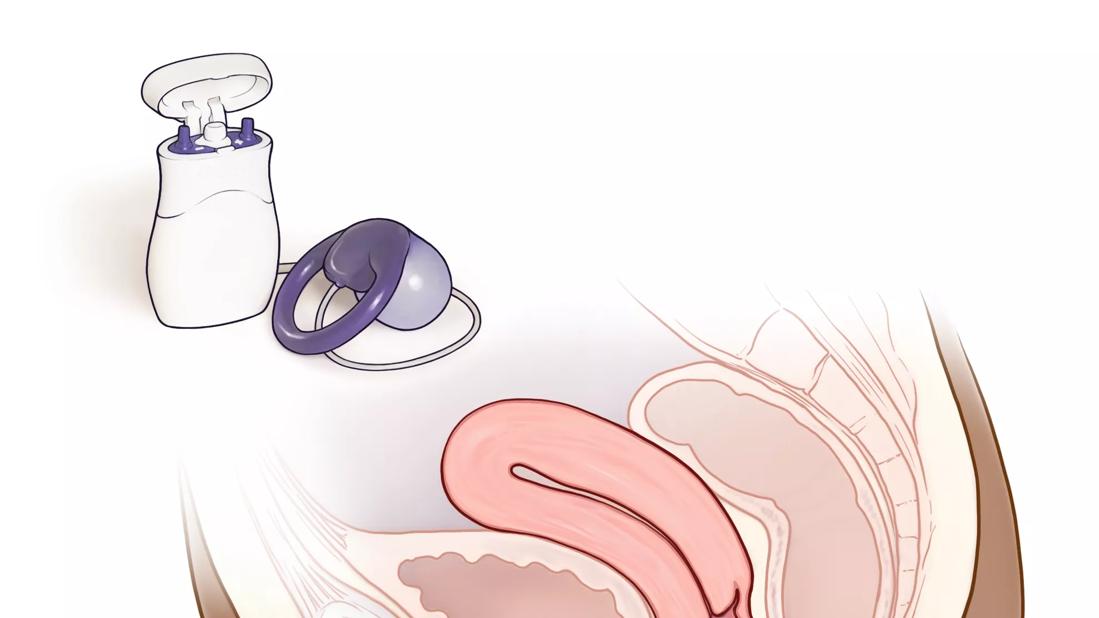
The Eclipse System features a vaginal insert, tubing and detachable inflation pump. The insert is a dual-layer balloon with a silicone surface and a polyurethane bladder that’s held in place with a flexible base made of silicone and stainless steel. A tube, which is connected to a pressure sensor, descends out of the vagina and attaches to the pump. When inflated, the pump presses on the rectum to prevent fecal incontinence. The patient squeezes the pump three times to deflate the balloon and have a bowel movement. The pump can then be reinflated.
Advertisement
“It is similar to a vaginal pessary for prolapse,” says Dr. Wallace. Contracted urogynecologists and colorectal surgeons customize the pressure, balloon and base size to each patient, who receive a trial device for a few weeks to ensure the right fit. Once they receive their personal device, patients can insert it themselves and take it out routinely to clean it or opt for their physician to do so during office visits.
In a pivotal study of the system, women who had a minimum of four fecal episodes over two weeks were fitted with the device. The success rate at three months was 86.4%.
Like any treatment option, the vaginal insert is not indicated for all patients with fecal incontinence. Common causes of anal incontinence include obstetric trauma, surgical trauma and pelvic floor denervation. Dr. Wallace notes that an evaluation of fecal incontinence is key to determining the best treatment and recommends this step-by-step approach:
Advertisement
“If your patient reports fecal incontinence, it warrants a consultation with a colorectal surgeon or urogynecologist to have an appropriate workup,” says Dr. Wallace. “Once that workup is done and they have tried conservative therapy, then the Eclipse is a reasonable option to avoid surgery.” She adds that patients with no prolapse and normal to high sphincter strength and whose symptoms affect their quality of life are good candidates for the Eclipse System.
Today, there are several options for treating fecal incontinence, from conservative management to the vaginal balloon insert and surgeries, such as sphincteroplasty, end-to-end anastomosis and sacral neuromodulation. It’s incumbent on primary care physicians and gynecologists to guide patients toward a solution.
“As women get older, they are told that urinary and fecal incontinence are part of life. But I think there’s been a paradigm shift happening in the last decade or so,” says Dr. Wallace. “Physicians should ask their patients about incontinence and advocate for the best treatment options.”
Advertisement
Advertisement

Uterine transposition cleared the field for radiation therapy

ACOG-informed guidance considers mothers and babies

Prolapse surgery need not automatically mean hysterectomy

Artesunate ointment shows promise as a non-surgical alternative

New guidelines update recommendations

Two blood tests improve risk in assessment after ovarian ultrasound

Recent research underscores association between BV and sexual activity

Psychological care can be a crucial component of medical treatment