By Tarek Souaid, MD; Jonathan Taliercio, DO; James F. Simon, MD, MBA; Ali Mehdi, MD, MEd; and Georges N. Nakhoul, MD, MEd
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Editor’s note: This article was originally published in the Cleveland Clinic Journal of Medicine and was reproduced with permission from the journal. Access the original article here.
Anemia is a common complication of chronic kidney disease. Its prevalence increases as the glomerular filtration rate decreases,1 and most patients with a rate lower than 30 mL/min/1.72 m2 develop anemia.2 Older studies linked anemia of kidney disease to numerous complications, including the onset and progression of left ventricular hypertrophy, heart failure, decreased quality of life, faster progression of chronic kidney disease, and death.3–6
Subsequently, several randomized controlled trials shed light on the benefits and limitations of iron supplements and erythropoiesis-stimulating agents (ESAs). This allowed the development of iron supplementation and hemoglobin targets. In addition to traditional ESAs, newer therapies have been developed and are now being used in clinical practice.
In this article, we discuss the pathophysiology of anemia of chronic kidney disease, the major clinical trials, and novel therapies.
Anemia of chronic kidney disease is multi-factorial and can be caused by decreased erythropoietin production by the peritubular interstitial cells of the kidney, higher circulating levels of uremia-induced inhibitors of erythropoietin, shorter red blood cell life span, and relative iron deficiency.7 However, measuring serum levels of erythropoietin is of no diagnostic utility in patients with anemia of chronic kidney disease, as there is no clear threshold defining a low value, especially in uremia, which can induce resistance to erythropoietin.8,9
Advertisement
Absolute iron deficiency results from decreased iron stores, whereas functional iron deficiency is characterized by sufficient iron stores but inadequate incorporation of iron into erythroid precursors.10 Hepcidin, a liver peptide and crucial regulator of iron homeostasis, is thought to play a key role in this process. Mobilization of iron into the circulation from enterocytes, iron-recycling macrophages, and hepatocytes requires transport across the cellular membrane by a transporter called ferroportin.11 Binding of hepcidin to ferroportin degrades the channel and inhibits iron mobilization out of the cell.
Normally, hepcidin levels decrease in conditions such as anemia, absolute iron deficiency, and hypoxia, in which more iron is needed for erythropoiesis. Conditions of systemic inflammation, such as chronic kidney disease, result in elevated hepcidin levels, diminished iron mobilization, and relative iron deficiency.12,13 The result is less iron available for erythropoiesis, with resistance to the action of ESAs (Figure 1).14–16
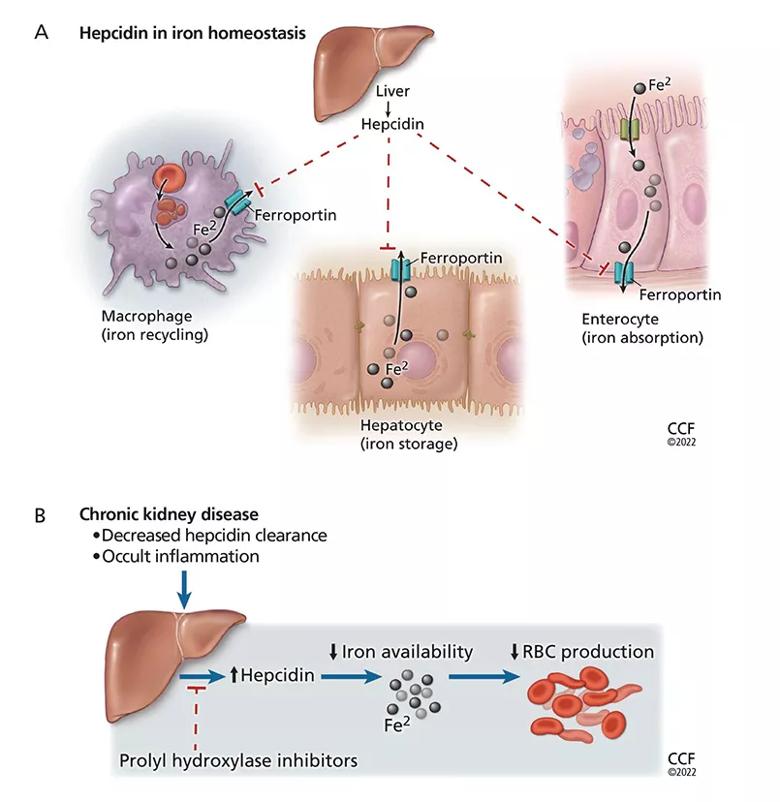
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a1eb8c6c-0947-4eaa-8a91-74d2330c3f20/22-URL-3106652-inset-2_jpg)
Figure 1. Hepcidin limits erythropoiesis. (A) Hepcidin plays a key role in iron homeostasis. It is produced by the liver and acts to degrade the iron transporter ferroportin, thus preventing release of iron (Fe2) from enterocytes, hepatocytes, and macrophages into the circulation. (B) In chronic kidney disease, hepcidin levels are elevated as a result of the underlying occult inflammatory state and as a result of decreased renal clearance of hepcidin. This makes less iron available for erythropoiesis and can lead to resistance to erythropoiesis-stimulating agents. Prolyl hydroxylase inhibitors (PHIs) decrease liver production of hepcidin, which may improve iron metabolism and lead to efficient management of anemia of chronic kidney disease. (RBC = red blood cell.)
Advertisement
Before erythropoietin was discovered, anemia of chronic kidney disease was treated with red blood cell transfusions and androgens. Frequent red blood cell transfusions led to iron overload, transmission of viral infections, and allosensitization to human lymphocyte antigens, with potential adverse effects on transfusion responsiveness and importantly, transplant candidacy and outcomes. Androgen therapy was the only potentially transfusion-sparing option, and it was used in the 1970s.17
After approval of the first of the ESAs by the US Food and Drug Administration (FDA) in 1989, studies continued to demonstrate benefits of the drugs. In a study published in 1990, the ESA recombinant human erythropoietin showed promising results, raising hemoglobin levels by more than 5 g/dL and keeping them there after 2 months of therapy in 10 patients on hemodialysis whose mean baseline hemoglobin level was 6.3 g/dL.18 A year later, a double-blind, randomized placebo-controlled trial in 118 patients on hemodialysis with hemoglobin levels less than 9 g/dL found improvements in quality of life and exercise capacity.19
By 2006, ESAs had become the treatment of choice of renal anemia, along with oral and intravenous iron supplementation, and were being given to most patients on dialysis in the United States.20
However, then came four randomized controlled trials21–24 using ESAs to raise hemoglobin levels to higher vs lower target levels in patients with chronic kidney disease, some of whom were on dialysis and some not. Except for reducing the number of transfusions needed and perhaps improving quality of life (the results differed), the trials found no benefits of treating to higher targets, and indeed highlighted higher risks of cardiovascular and cerebrovascular events and worsening of cancer outcomes (Table 1).21–25
Advertisement
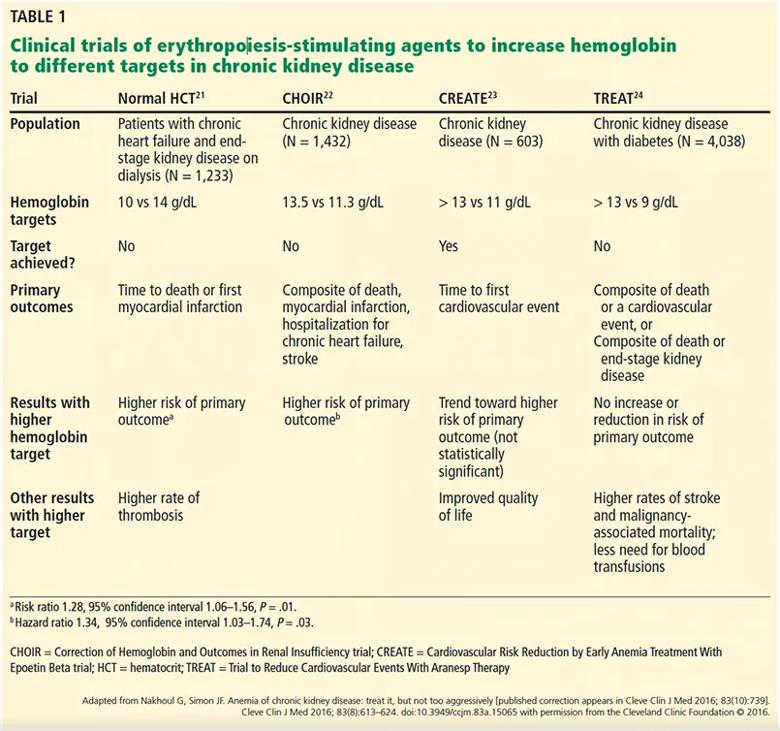
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b59b0067-c22d-4691-a33f-944dc46d3c51/22-URL-3106652-inset-Table-1_jpg)
Table 1. Clinical trials of erythropoiesis-stimulating agents to increase hemoglobin to different targets in chronic kidney disease
In view of these results, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines26 strongly advised against trying to raise hemoglobin to normal levels (eg, higher than 13.5 g/dL in men, 12 g/dL in women), instead recommending a target of 10 to 11.5 g/dL in patients with end-stage kidney disease. The guidelines also advised that the decision to start ESAs should be individualized for patients with chronic kidney disease with a hemoglobin level less than 10 g/dL.26
In addition, a closer look at the data identified a “hyporesponsive” group in whom ESAs failed to achieve a higher hemoglobin target despite increasing doses. Interestingly, patients who received higher doses of ESAs had a higher rate of adverse events.27,28 Therefore, the KDIGO guidelines recommend using the lowest ESA doses needed to achieve the hemoglobin goal, realizing the only proven benefit is avoiding transfusions.29 Other possible benefits (again, the data conflict) include lessening of anemia-related symptoms, higher quality of life,30–32 and reduction in left ventricular hypertrophy.33 Table 2 summarizes the potential risks and benefits of ESA therapy.18,21–24,29–33
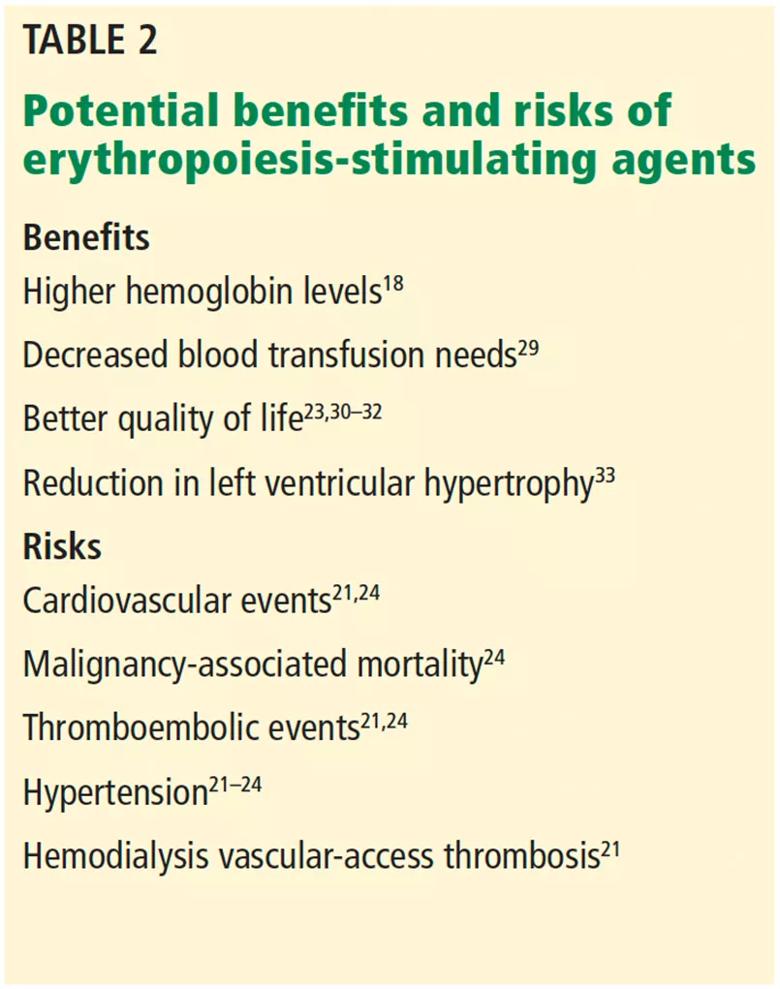
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8ead1b55-10e9-46df-845f-4199ccce5830/22-URL-3106652-inset-Table-2_jpg)
Table 2. Potential benefits and risks of erythropoiesis-stimulating agents
Commonly used ESAs are recombinant human erythropoietin (epoetin alfa), darbepoetin alfa (which has a longer half-life),34,35 and a recently approved form of pegylated recombinant human erythropoietin (Table 3).36–39
Advertisement
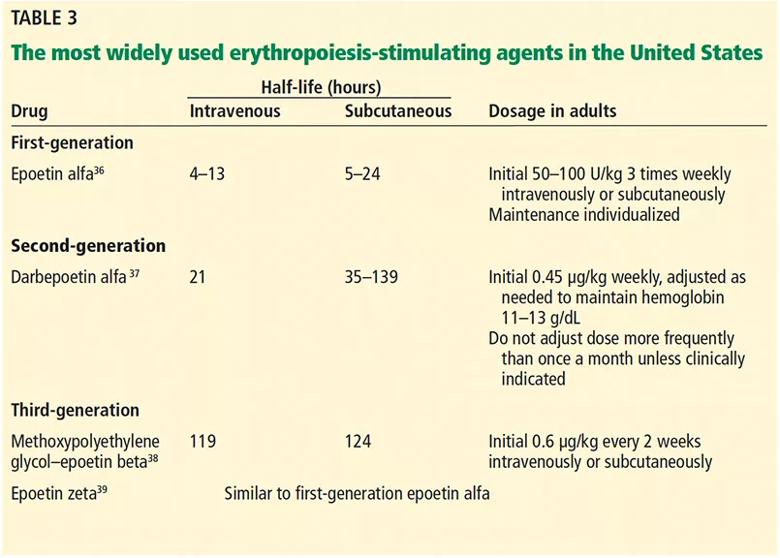
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ce5acbc7-3a02-4a36-a341-4a5a0f7f6d6e/22-URL-3106652-inset-Table-3_jpg)
Table 3. The most widely used erythropoiesis-stimulating agents in the United States
ESAs continue to be the mainstay in managing anemia of chronic kidney disease. The newer ESAs were developed by increasing the glycosylation or pegylation of recombinant human erythropoietin to prolong its half-life and affinity for the erythropoietin receptor. However, their mechanism of action is essentially the same, and they carry the same potential risks, ie, adverse cardiovascular events, thrombosis, stroke, and poor cancer outcomes.
The hypoxia-inducible factor (HIF) pathway was a revolutionary discovery, and drugs that target it promise to improve outcomes in diseases that include anemia of chronic kidney disease.
HIF is a heterodimer consisting of an alpha and a beta subunit. Heterodimerization of the two subunits activates transcription of numerous genes and regulates a multitude of biologic and metabolic processes such as angiogenesis, cell growth and differentiation, and erythropoiesis.40,41 The transcriptional activity of HIF is controlled primarily by its degradation rate.42
Stable HIF leads to more erythropoietin
While the beta subunit of HIF is constitutively expressed (ie, it is present all the time), the alpha subunit is regulated by oxygen tension. At normal oxygen levels, the proline residue of HIF alpha is hydroxylated (ie, receives a hydroxyl [OH–] group) by the enzyme prolyl hydroxylase, leading to ubiquitylation (inactivation by binding to ubiquitin) and proteasomal degradation.43 This impedes formation of alpha-beta heterodimers, preventing erythropoietin transcription. The opposite happens in hypoxic states, in which HIF alpha is no longer degraded, and HIF heterodimers bind to the hypoxia-response elements, increasing erythropoietin transcription.
Prolyl hydroxylase inhibitors (PHIs), also known as HIF stabilizers, prevent hydroxylation of HIF alpha and its subsequent degradation. Thus, they stabilize the HIF alpha-beta heterodimer and enhance downstream expression of the erythropoietin gene EPO, leading to increased endogenous erythropoietin production (Figure 2).
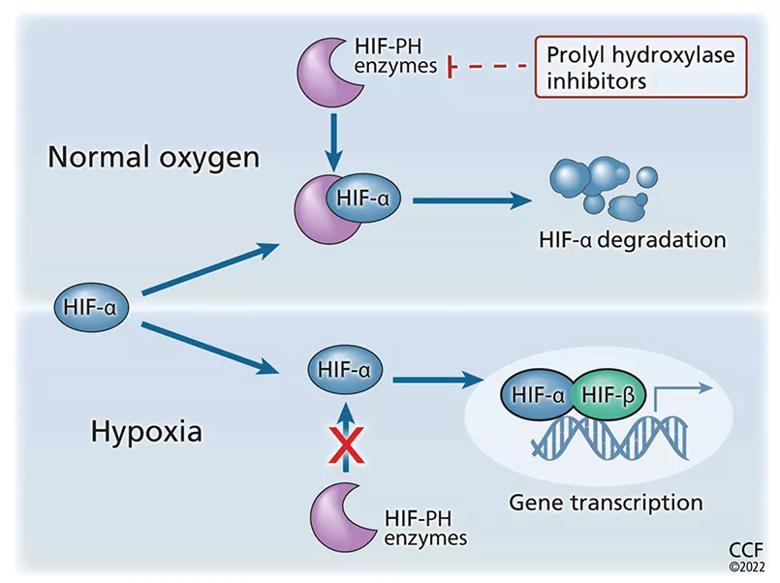
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9238cffe-ce65-4c4d-a2cd-520961aaa54b/22-URL-3106652-Inset-1-1_jpg)
Figure 2. Prolyl hydroxylase (PH) inhibitors increase erythropoiesis. Hypoxia-inducible factor (HIF) is a heterodimer consisting of 2 subunits, alpha and beta (HIF-α, HIF-β). Undernormal oxygen tension, the proline residue of HIF-α is hydroxylated and subsequently degraded. This prevents the formation of the heterodimer and stops subsequent erythropoietin transcription. When oxygen tension is low, HIF-α is not degraded, thus allowing HIF heterodimers to form. This leads to an increase in erythropoietin transcription. By preventing hydroxylation of HIF-α, PHIs prevent its degradation and stabilize the HIF-α/HIF-β heterodimer. This leads to increased downstream gene expression with increased production of endogenous erythropoietin.
In 2015, a phase 2 randomized clinical trial compared an oral PHI (roxadustat) with placebo in patients with anemia of chronic kidney disease not on dialysis who had never received an ESA.44 An effective hemoglobin increase from baseline (arbitrarily defined as ≥ 1 g/dL) occurred in all 20 of the 20 patients receiving roxadustat 2 mg/kg 2 to 3 times per week compared with 3 (13%) of the 23 patients receiving placebo. Notably, roxadustat raised endogenous erythropoietin and lowered hepcidin levels.
In 2019, in an 18-week, double-blind, randomized, open-label, phase 3 trial in China in 154 patients with anemia of chronic kidney disease who were not on dialysis, those receiving roxadustat had a higher mean hemoglobin level than those receiving placebo after 8 weeks, with maintained efficacy afterwards.45 In this study, roxadustat reduced cholesterol levels.
In 2020, in the ANDES study,46 a phase 3 global randomized clinical trial in 916 anemic patients with nondialysis-dependent chronic kidney disease (analyzed by an intention-to-treat model), oral roxadustat given 3 times per week was superior to placebo in hemoglobin correction and maintenance, with the same overall tolerability.
In OLYMPUS,47 another recent phase 3 randomized clinical trial, in 2,781 patients, roxadustat significantly raised hemoglobin levels and decreased the need for blood transfusions, with a side-effect profile similar to that of placebo.
Additionally, the ALPS study,48 a phase 3, multicenter, randomized, double-blind, placebo-controlled trial in 594 patients, found that roxadustat was superior to placebo with regard to hemoglobin response rate and change in hemoglobin level from baseline, with a comparable adverse-event profile.
A pooled analysis of the ANDES, OLYM-PUS, and ALPS trials concluded that roxadustat was effective at increasing hemoglobin levels in anemic patients with chronic kidney disease not on dialysis, reduced the need for blood transfusions, and did not demonstrably increase the risk of major adverse cardiac events.49,50
The first phase 2 clinical trial of oral PHI therapy to report hemoglobin correction in patients on dialysis who had never received ESAs, published in 2016, was an open-label study of roxadustat (with no placebo group).51 The mean hemoglobin level increased by at least 2.0 g/dL within 7 weeks, independent of initial hemoglobin level, iron-repletion state, iron supplementation, C-reactive protein level, and dialysis type (hemodialysis or peritoneal), and hepcidin concentrations decreased. This was the first study to challenge the historical notion that anemia in dialysis-dependent chronic kidney disease was a result of loss of the cells that produce endogenous erythropoietin. Instead, it showed that the mechanism remains intact but was suppressed in the uremic milieu.
In 2019, a randomized phase 3 study of 305 patients in China with dialysis-dependent chronic kidney disease on ESAs found that oral roxadustat was noninferior to intravenous epoetin alfa.52 A notable result also seen in the 2016 phase 2 trial 51 was that inflammation (assessed by C-reactive protein levels) did not affect the hemoglobin-correcting effect of roxadustat, whereas ESAs produce less of a response in inflammatory states.53
In 2021, the HIMALAYAS study 54 explored the efficacy and safety of roxadustat in a phase 3 open-label trial using epoetin alfa as the control treatment in 1,043 patients with anemia who had never received an ESA before and who had recently started on dialysis (from 2 weeks up to 4 months before randomization). Roxadustat was noninferior to epoetin alfa in correcting and maintaining hemoglobin levels, with comparable adverse-event rates with either treatment.54
PHIs are promising drugs, as they are at least as effective as ESAs in raising hemoglobin levels in patients with chronic kidney disease either on or off dialysis. They increase iron availability by decreasing hepcidin levels, thus potentially reducing the need for intravenous iron. Most importantly, they seem to remain effective in inflammatory states. This is a striking difference from ESAs, which require higher doses in inflammatory states and often fail to achieve target hemoglobin values.21,22,24 This ESA resistance and the higher required doses are likely responsible for the negative cardiovascular outcomes in hyporesponsive patients.27,28
Additional advantages of PHIs include ease of administration (oral dosing, especially advantageous in patients not on dialysis or who are on home hemodialysis), cheaper manufacturing with less stringent transportation logistics (ESAs need to be kept refrigerated),55 and less immunogenicity (a concern with ESAs), given that they are not protein-based. Also, roxadustat seemed to lower cholesterol levels, though the mechanism remains to be understood.45
Adverse effects of PHIs
So far, no major serious adverse effects have been found in clinical trials. The serious adverse events that happened in phase 2 trials were not considered drug-related or were deemed “within expected range,” as they happened at comparable rates in comparable patients who were not on these drugs.56 However, studies of the PHI FG-2216 were suspended after a patient died of hepatic necrosis, making it the only drug of this class for which a study was halted due to safety concerns.57
Hyperkalemia. The two Chinese phase 3 trials45,52 showed an increased risk of hyperkalemia with roxadustat. Hyperkalemia has also been reported in trials of daprodustat in patients on hemodialysis.58 Thus, use of PHIs in patients predisposed to hyperkalemia should be approached with caution.
Upper respiratory tract infections and metabolic acidosis have also been reported. More side effects may emerge with larger and longer studies.
Cardiovascular safety. A pooled post hoc analysis compared roxadustat with epoetin alfa in 1,530 patients newly started on dialysis.49 Although the results suggested a lower risk of death, myocardial infarction, and stroke in the roxadustat group, an analysis with prespecified stratification factors had shown attenuated benefits and wider confidence intervals,59 leading to the results of this study being called into question.60 Thus, the cardiovascular superiority of PHIs is yet to be fully established.
Theoretical risk of malignancy. To date, no animal or human study has shown PHIs to increase the risk of kidney cancer or other malignancies. However, HIF activation may enhance the proliferation, invasion, and metastatic potential of cells, as HIFs appear to be linked to metastasis in several malignancies (breast, prostate, lung, bone, and colorectal cancer).61
Diabetic retinopathy (either its induction or progression) is another potential adverse effect. HIF-1 alpha can induce vascular endothelial growth factor, which is involved in the progression of this disease. No such cases have been reported, but because many trials excluded patients at high risk of retinal hemorrhage, PHIs should be used with caution in this population.42
Progression of autosomal dominant polycystic kidney disease. In theory, PHIs could raise the risk of cyst growth, since HIF expression levels have been correlated in human and rat models with increased cyst burden.62 Until better evidence is available, some experts suggest limiting PHI use in patients with this disease who are not on dialysis.63
Roxadustat is currently approved for use in anemia of chronic kidney disease in nondialysis-dependent and dialysis-dependent patients in China, Japan, and Chile, and recently received a positive opinion by the Committee for Medicinal Products for Human Use of the European Medicines Agency.64
However, concerns about its cardiovascular safety prompted the FDA Cardiovascular and Renal Drugs Advisory Committee to vote against the approval of roxadustat in July 2021. In August 2021, the FDA responded to roxadustat’s New Drug Application by asking for a new clinical trial to assess the safety of this drug in both nondialysis-and dialysis-dependent chronic kidney disease.64 Therefore, it seems unlikely that roxadustat will be available in the United States in the immediate future.
Although other PHIs have been approved for clinical use in Japan,65 roxadustat is the most studied to date. The 14 published phase 2 and 3 trials of roxadustat44–48,51,52,54,66–71 are summarized in Table 4.
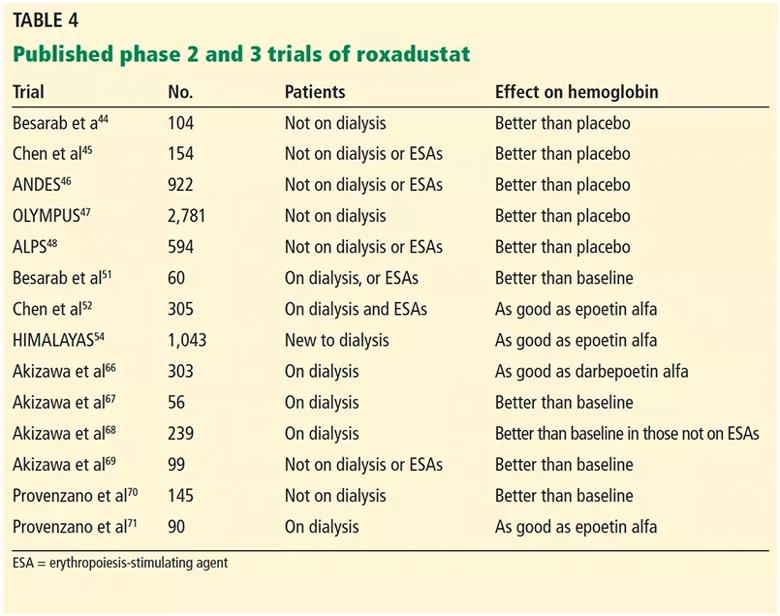
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3c7e3a59-d841-4a4c-bf95-3cece0e9ae09/22-URL-3106652-inset-Table-4-1_jpg)
Table 4. Published phase 2 and 3 trials of roxadustat
More research is needed to answer the following important questions about PHIs:
However, regardless of the lingering questions, the exploration and exploitation of the HIF pathway opens the door to multiple possibilities. Will PHIs deliver on the promises? We certainly hope so. It would be great to have one more tool at our disposal to treat anemia in patients with kidney disease.
References
Advertisement

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Fixed-dose single-pill combinations and future therapies

Reproductive urologists publish a contemporary review to guide practice

Two recent cases show favorable pain and cosmesis outcomes

Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood

Proteinuria reduction remains the most important treatment target.

IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy

Oncologic and functional outcomes are promising, but selection is key