A case-based review of a condition more prevalent than once thought
By Kaylee Watson, MD; David Wolinsky, MD; Mauricio Velez, MD; and David Snipelisky; MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Note: This article is reprinted from the Cleveland Clinic Journal of Medicine (2023;90[2]:95-101).
A 50-year-old man with a history of bilateral carpal tunnel syndrome presented with progressive shortness of breath on exertion for the previous three months. Cardiovascular physical examination showed jugular venous distention to the middle neck at 30 degrees, a palpable maximal impulse displaced inferiorly and laterally with a prominent S4 heart sound, and bilateral bronchovesicular lung sounds. Tinel sign was present, as noted by a tingling sensation following percussion over the median nerve on both wrists. Further investigation revealed a serum creatinine level of 1.4 mg/dL (reference range, 0.74-1.35) with undetectable troponin T enzyme, elevated N-terminal-pro-brain natriuretic peptide (NT-proBNP) of 2,145 pg/mL (reference range, < 125 pg/mL), and ECG with low voltage, normal sinus rhythm, and Q waves in the anterior leads.
1. Which of the following diagnostic studies is the most appropriate to obtain next?
The initial differential diagnosis for a patient who presents with dyspnea on exertion, abnormal ECG findings and negative cardiac enzymes includes heart failure, myocardial ischemia, pericardial effusion and noncardiac etiologies.1
Chest radiography would be beneficial to determine lung involvement and evaluate cardiac silhouette to screen for any abnormalities. However, cardiovascular abnormalities seen on chest radiography, such as altered shape or widened mediastinum, would be nonspecific in this patient, and there are better imaging studies for heart function and anatomy. Additionally, in the context of abnormal physical examination findings — including abnormal heart sounds, elevated jugular venous pressure and NT-proBNP elevation — a cardiac manifestation is more likely.
Advertisement
The cardiac stress test is a common diagnostic modality for patients with chest pain who are at risk for obstructive coronary artery disease. The patient’s lack of cardiovascular risk factors and younger age make ischemia less likely, particularly if the chest discomfort is not thought to be an angina equivalent. Similar reasoning can be used as to why a coronary angiogram would not be the ideal initial study to obtain. The patient has Q waves on the ECG, yet although an ischemic etiology is imperative to remain within the differential, in the context of no other findings suggestive of angina-equivalent symptoms, coronary angiography would not be the next step in management.
The best diagnostic study for this patient would be an echocardiogram to accurately assess the structure and function of the patient’s heart. This will allow the clinician to narrow the potential reasons for the patient’s presentation. According to the American College of Cardiology/American Heart Association appropriate use criteria, there are numerous indications for an echocardiographic evaluation, including suspicion of heart failure, as in our clinical presentation.2
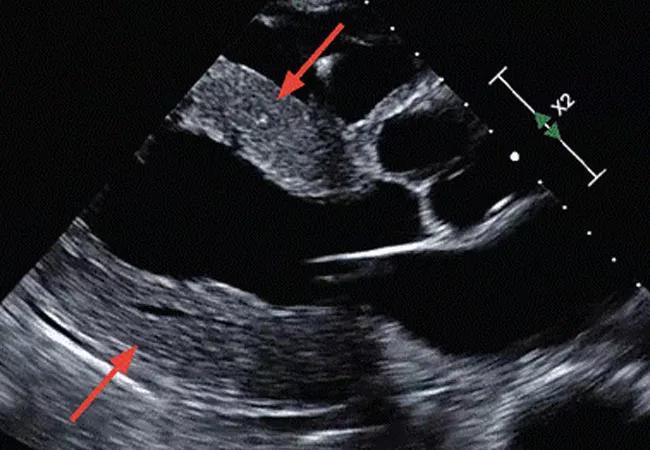
Echocardiography showed a left ventricular ejection fraction of 55% and concentric left ventricular wall hypertrophy with a wall thickness of 15 mm (Figure 1). Echocardiography with strain imaging to evaluate function of the myocardium revealed longitudinal impairment with apical sparing, i.e., the “cherry-on-top” appearance. There was no pericardial effusion.
Advertisement
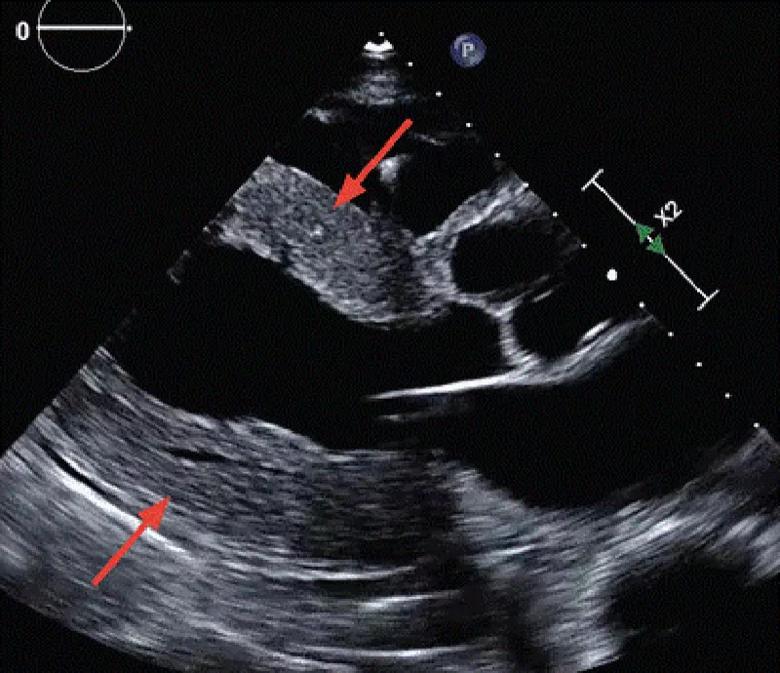
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b2435ced-66be-4789-b849-d362d7fd1ed9/23-CCC-3884461-CQD-Inset1-800x690-1_jpg)
Figure 1. Parasternal long-axis view on echocardiography demonstrates diffuse concentric left ventricular hypertrophy (arrows).
At this time, infiltrative and hypertrophic pathologies should be considered (Table 1), such as Fabry disease, Danon disease, mucopolysaccharidosis and hypertrophic cardiomyopathy.1 However, in the context of this patient’s presentation it is important to consider cardiac amyloidosis, a type of infiltrative cardiomyopathy.
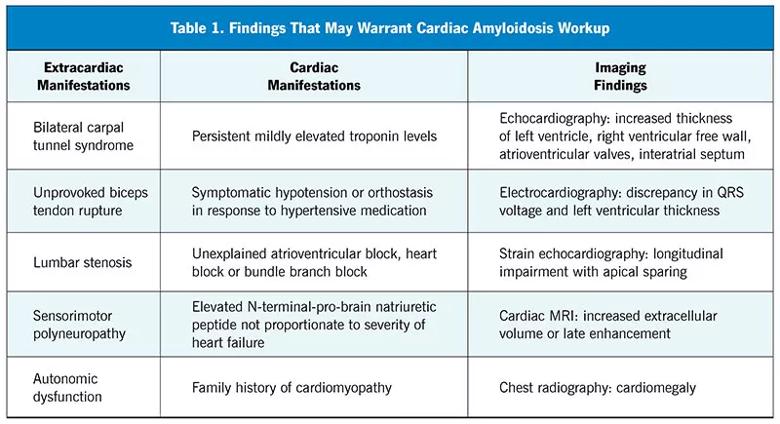
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/eb74c582-ffce-4855-a2ec-f69df60dbc02/23-CCC-3884461-CQD-Inset-Table1-800x435-1_jpg)
In this patient, red flags for cardiac amyloidosis include the ECG findings accompanied by a history of carpal tunnel syndrome and evidence of renal dysfunction.1 ECG findings generally include a discordance in ECG voltage, which may be reduced or normal, and the degree of hypertrophy on imaging. Cardiac amyloidosis typically demonstrates abnormal global longitudinal strain with apical sparing, which helps differentiate this disease process from other etiologies of hypertrophy.3 A history of carpal tunnel syndrome is common in patients with systemic amyloidosis as protein deposition can occur in both cardiac and noncardiac organ systems.1
Amyloidosis is an infiltrative disease due to the accumulation of misfolded precursor proteins that make up amyloid.1,4,5 Two major types include light chain amyloidosis (AL) and transthyretin amyloidosis (ATTR), which is further subdivided into hereditary (hATTR) and wild-type (ATTRwt). While cardiac amyloidosis historically has been thought to be a rare disease, emerging imaging and other advancements have revealed a greater prevalence of ATTR than previously believed. This may be due to the phenotypic heterogeneity in presentation of the disease.1,4,5 A survey of patients with ATTR and their caregivers showed that 57% of patients with hATTR and 39% of patients with ATTRwt received a misdiagnosis, 17% sought care from five different physicians before proper diagnosis, and those who were misdiagnosed received treatment for the wrong disease 75% of the time.4
Advertisement
Patients typically do not receive appropriate care owing to similarities of clinical presentation of cardiac amyloidosis with other etiologies of heart failure, the relatively advanced age of the patient population, misconceptions pertaining to the disease process and common misdiagnosis.1,4,5 Arrhythmias and bilateral carpal tunnel syndrome commonly precede heart failure symptoms by many years in patients with transthyretin amyloid cardiomyopathy (ATTR-CM).1,6,7 It is also important to identify the myriad noncardiac manifestations associated with the disease. For instance, patients tend to have a degree of renal dysfunction, neuropathies and tendinopathies, including unprovoked tendon rupture, autonomic dysfunction,8 lumbar spinal stenosis,9 need for early joint replacements, issues with bowel motility10 and even retinal deposition of the misfolded protein.1,5 A thorough history from the patient and care team along with a high suspicion for ATTR-CM is essential for piecing together the diagnosis.
2. What diagnostic studies should be performed early in the suspicion of cardiac amyloidosis?
In a patient with high suspicion for cardiac amyloidosis, the clinical priority is differentiating between an etiology of AL or ATTR, and genetic testing would not provide a definitive diagnosis.5,11 Genetic testing is warranted once ATTR is discovered to determine whether the form of disease is hATTR or ATTRwt5; hATTR indicates that first-degree relatives are at higher risk for development of this pathology.5 Fat-pad biopsies can be used to evaluate for amyloid deposition, but the sensitivity for ATTR is approximately 50%, leading to unnecessary pain for the patient and potential false-negative results.5,12 Endomyocardial biopsy would be warranted after conflicting or equivocal findings of another less-invasive test.5,12
Advertisement
Two of the primary types of systemic amyloidosis include ATTR amyloidosis, in which the liver produces excess protein that ultimately misfolds and deposits in various tissues, and AL amyloidosis, which is primarily a bone marrow dyscrasia in which monoclonal proteins are overproduced and deposit in various tissues.1,5 These two conditions can have similar presentations, but their treatment pathways are completely different, so it is of the utmost importance to rule out AL early.5,11-13 With a six-month median survival of patients from time of diagnosis with AL, an urgent hematology-oncology evaluation is warranted.13 Sensitivity of over 99% for AL is achieved when combining serum immunofixation electrophoresis, urine immunofixation electrophoresis and serum light chain concentration.12 Serum plasma electrophoresis has an inferior sensitivity of approximately 70% and should be avoided.12 These three tests should be ordered before or simultaneously with diagnostic imaging to avoid delay of targeted treatment.
Amyloidosis involves the misfolding and subsequent deposition of precursor proteins, infiltrating numerous organ systems.5,11-13 Transthyretin is a tetramer that acts as a tertiary carrier protein for thyroxine and holo-retinol binding protein and is mostly secreted from the liver into the blood.12 In hATTR, a single amino acid mutation occurs on chromosome 18 where the transthyretin gene is found, causing aggregation to be more efficient.5,11,12 In ATTRwt, the wild-type protein becomes unstable without any evidence of damage to the genetic sequence.5,11
3. What is the next step in confirming suspicion of ATTR?
Cardiac MRI would be beneficial in this patient if echocardiography was inconclusive and further investigation was warranted. Cardiac MRI would not give a definitive diagnosis for ATTR but may be useful when excluding other pathologies, such as hypertrophic cardiomyopathy or a different infiltrative process. It can be suggestive of amyloidosis but not diagnostic. FDG-PET is used to detect sarcoidosis or malignancy but would not be useful in the diagnosis of cardiac amyloidosis.
Although endomyocardial biopsy still has a role in certain clinical conditions, it would be an invasive and more aggressive modality to help make the diagnosis compared with more conventional imaging modalities.
Technetium-99m pyrophosphate scintigraphy is the recommended diagnostic tool for ATTR-CM. It is cost-effective, noninvasive and relatively widely available.14,15 The radiotracer used is generally absorbed by bone structures and amyloid deposition in the myocardium, and therefore the degree of uptake in the myocardium is generally compared with that of the contralateral ribs.14,15 In normal myocardium, no uptake would be present, but in patients with ATTR-CM, radiotracer uptake is usually comparable to or exceeds that of the contralateral ribs based on severity of the disease process. Due to the diffuse deposition of amyloid throughout the myocardial tissue, the sensitivity of endomyocardial biopsy for the diagnosis of cardiac amyloidosis is nearly 100%.14 Nonetheless, the risks of the procedure and limited access make it the less favorable option. When observing 103 patients undergoing diagnostic endomyocardial biopsy for ATTR-CM, a radiotracer uptake of grade 2 to 3 had sensitivity of 94% and a specificity of 89%, with 100% specificity for grade 3.15
While false-positive results may occur with this test, further investigations are recommended to diminish this likelihood.14,15 To distinguish blood pooling from myocardial uptake, single-photon emission computed tomography is necessary after technetium-99m pyrophosphate scintigraphy has shown evidence of ATTR-CM.12,15 Once blood pooling and AL amyloidosis have been ruled out, ATTR-CM will meet diagnostic criteria if scintigraphy shows grade 2 to 3 cardiac uptake three hours after injection, as seen in our patient (Figure 2).14 ATTR should only be established after AL has been ruled out with serum immunofixation electrophoresis, urine immunofixation electrophoresis and serum light chain concentration.12,15
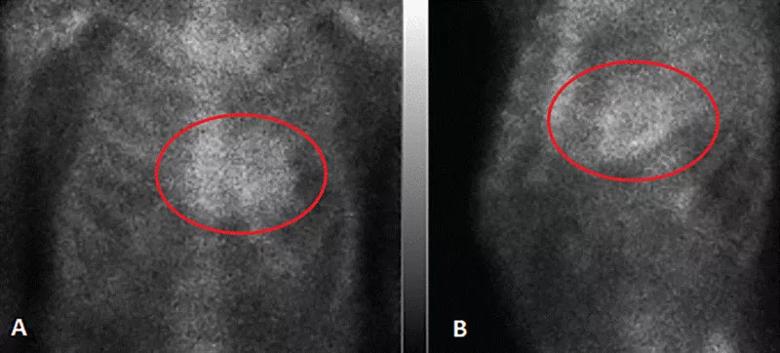
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1102af2b-e867-402d-80b0-e3725f5a9b06/23-CCC-3884461-CQD-Inset2-800x362-1_jpg)
Figure 2. Technetium-99m pyrophosphate scintigraphy. (A) Anterior and (B) left anterior oblique views in our patient demonstrated grade 2 to 3 myocardial uptake of the radiotracer (circles) three hours after injection, thus meeting diagnostic criteria for transthyretin amyloid cardiomyopathy.
4. What is the best disease-modifying medical treatment for cardiac amyloidosis?
Although doxycycline and NSAIDs have been used historically in the treatment of cardiac amyloidosis, there are no data to support their ability to alter the disease process or to show a survival benefit.5,12 These treatments were used on the premise of having efficacy in other inflammatory and infectious cardiac etiologies.5 Inotropes and diuretics may improve quality of life for patients with specific types of cardiomyopathies but will not change disease progression.16 The pathogenesis of ATTR-CM involves the deposition of proteins. Therefore, these methods would not alter the disease course.
The goal of drugs that target ATTR is to slow progression of the disease. However, cardiac and extracardiac manifestations of ATTR must also be managed. Due to the low stroke volume of the heart in ATTR-CM, beta-blockers should be avoided, though they may be necessary for rate control of arrhythmias that commonly occur owing to atrial involvement of the disease.16 Referral to a neurologist may be necessary, as the patient may experience polyneuropathy and autonomic instability evidenced by orthostatic hypotension.16
Disease-modifying therapies for ATTR-CM target transthyretin through silencing, stabilization and disruption (Table 2). Tafamidis is the first FDA-approved treatment for ATTR-CM and is used in both the wild-type and hereditary subtypes.17,18 Its mechanism is to stabilize the transthyretin protein in its tetrameric configuration, ultimately preventing breakdown into unstable monomers that infiltrate various organ systems. In the ATTR Tafamidis in Transthyretin Cardiomyopathy Clinical Trial,17 patients randomized to tafamidis not only had a decrease in cardiovascular-related hospitalizations but also showed less decline in functional capacity and reduced all-cause mortality. Over a 30-month span of treatment with tafamidis, therapy was tolerated well with safety profiles similar to those with placebo.17 Furthermore, patients randomized to tafamidis experienced less worsening of their general health, reduced or no worsening in heart failure symptoms, and improved quality of life.18 Other transthyretin-stabilizing drugs such as diflunisal have been shown to improve survival in patients with cardiac ATTR.19 Further research targeting ATTR through silencing and disruption shows promising results: medications like patisiran and inotersen have reduced production of transthyretin by up to 80%, ultimately stabilizing or partially relieving peripheral neuropathy.20
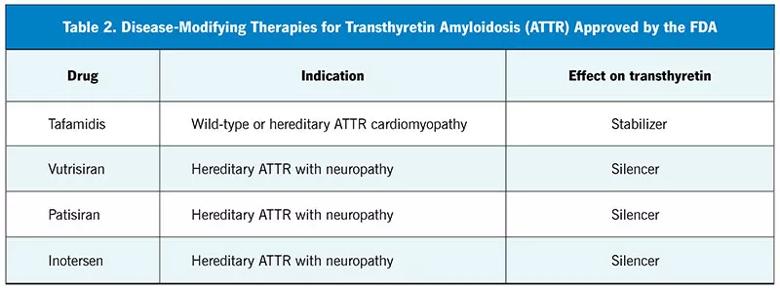
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/25eb107e-28f8-4a3c-915b-110547d24746/23-CCC-3884461-CQD-Inset-Table2-800x297-1_jpg)
5. This patient was started on tafamidis therapy. Two years later he was noted to have a reduced left ventricular ejection fraction of 35%, small left ventricle cavity size, worsening heart failure symptoms and a negative coronary angiogram. What treatment option should be considered?
Left ventricular assist devices (LVADs) are used for patients with end-stage heart failure with reduced ejection fraction. LVADs have demonstrated survival and quality-of-life benefits among select patient populations. Patients with cardiac amyloidosis tend to have small ventricle sizes as a result of hypertrophy, which will likely preclude successful LVAD implantation, and no study has demonstrated improvement in morbidity or mortality in this population.21 Therefore, LVAD therapy would not be an ideal option for our patient.
Diuretics are an adjunct to traditional guideline-directed therapies in patients with reduced ejection fraction. Although diuretics improve symptoms by way of decongestion, they do not increase survival and will not modify disease progression in heart failure with reduced ejection fraction. Hospice or palliative care may be an option for a patient with ATTR-CM who elects not to proceed with more invasive treatments or would not be able to tolerate them.
The patient’s age and lack of other significant comorbidities should warrant consideration for heart transplantation as the next step in management.
The patient was ultimately considered for and underwent successful orthotopic heart transplant without complications.
Dr. Wolinsky reports consulting for Alnylam Pharmaceuticals and Pfizer, as well as consulting, teaching and speaking for Akcea Therapeutics and Astellas Pharma US. The other authors report no relevant financial relationships that, in the context of their contributions, could be perceived as a potential conflict of interest.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. doi:10.1016/j.jchf.2019.04.010
2. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to update the 1997 guidelines for the clinical application of echocardiography). J Am Soc Echocardiogr. 2003;16(10):1091-1110. doi:10.1016/S0894-7317(03)00685-0
3. Nardozza M, Chiodi E, Mele D. Left ventricle relative apical sparing in cardiac amyloidosis. J Cardiovasc Echogr. 2017;27(4):141-142. doi:10.4103/jcecho.jcecho_22_17
4. Lousada I, Maurer M, Warner M, Guthrie S, Hsu K, Grogan M. Amyloidosis research consortium cardiac amyloidosis survey: results from patients with ATTR amyloidosis and their caregivers. Orphanet J Rare Dis. 2017;12(suppl 1):165. doi:10.1186/s13023-017-0710-5
5. Donnelly JP, Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84(12 suppl 3):12-26. doi:10.3949/ccjm.84.s3.02
6. González-López E, López-Sainz Á, Garcia-Pavia P. Diagnosis and treatment of transthyretin cardiac amyloidosis. Progress and hope. Rev Esp Cardiol (Engl Ed). 2017;70(11):991-1004. doi:10.1016/j.rec.2017.05.036
7. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404-2412. doi:10.1161/CIRCULATIONAHA.116.021612
8. Siddiqi OK, Ruberg FL. Cardiac amyloidosis: an update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28(1):10-21. doi:10.1016/j.tcm.2017.07.004
9. Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201-207. doi:10.1038/modpathol.2014.102
10. Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141-157. doi:10.1093/qjmed/91.2.141
11. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872-2891. doi:10.1016/j.jacc.2019.04.003
12. Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142(1):e7-e22. doi:10.1161/CIR.0000000000000792
13. Sperry BW, Ikram A, Hachamovitch R, et al. Effi cacy of chemotherapy for light-chain amyloidosis in patients presenting with symptomatic heart failure. J Am Coll Cardiol. 2016;67(25):2941-2948. doi:10.1016/j.jacc.2016.03.593
14. Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2 — evidence base and standardized methods of imaging. J Nucl Cardiol. 2019;26(6):2065-2123. doi:10.1007/s12350-019-01760-6
15. Poterucha TJ, Elias P, Bokhari S, et al. Diagnosing transthyretin cardiac amyloidosis by technetium Tc 99m pyrophosphate: a test in evolution. JACC Cardiovasc Imaging. 2021;14(6):1221-1231. doi:10.1016/j.jcmg.2020.08.027
16. Adam RD, Coriu D, Jercan A, et al. Progress and challenges in the treatment of cardiac amyloidosis: a review of the literature. ESC Heart Fail. 2021;8(4):2380-2396. doi:10.1002/ehf2.13443
17. Damy T, Garcia-Pavia P, Hanna M, et al. Effi cacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2021;23(2):277-285. doi:10.1002/ejhf.2027
18. Hanna M, Damy T, Grogan M, et al. Tafamidis and quality of life in people with transthyretin amyloid cardiomyopathy in the study ATTR-ACT: a plain language summary. Future Cardiol. 2022;18(3):165-172. doi:10.2217/fca-2021-0095
19. Rosenblum H, Castano A, Alvarez J, Helmke S, Maurer MS. Improved survival in patients with transthyretin cardiac amyloidosis treated with TTR stabilizers. J Card Fail. 2017;23(8):S20. doi:10.1016/j.cardfail.2017.07.039
20. Carroll A, Dyck PJ, de Carvalho M, et al. Novel approaches to diagnosis and management of hereditary transthyretin amyloidosis. J Neurol Neurosurg Psychiatry. 2022;93(6):668-678. doi:10.1136/jnnp-2021-327909
21. Mehra MR, Uriel N, Naka Y, et al. A fully magnetically levitated left ventricular assist device — final report. N Engl J Med. 2019;380(17):1618-1627. doi:10.1056/NEJMoa1900486
Dr. Watson is a fellow researcher in the Department of Cardiology and Cardiothoracic Surgery, Cleveland Clinic Florida. Dr. Wolinsky is Section Head of Nuclear Cardiology and Medical Director of the Cardiac Amyloid Center, Cleveland Clinic Florida. Drs. Velez and Snipelisky are both cardiologists in the Section of Heart Failure and Cardiac Transplant Medicine, Cleveland Clinic Florida.
Advertisement

Nonthermal technique reduces bleeding and perforation risk

Standardizing a minimally invasive approach for Barrett’s Esophagus and Esophageal Cancer

PSMA-targeted therapy for metastatic prostate cancer now offered at Cleveland Clinic Weston Hospital

Nationally recognized urologic oncologist offers vision for growth, innovation, and excellence

Noninvasive modality gains ground in United States for patients with early-to-moderate disease

Cleveland Clinic Weston Hospital’s collaborative model elevates care for complex lung diseases

Interventional pulmonologists at Cleveland Clinic Indian River Hospital use robotic technology to reach small peripheral lung nodules

Trained in the use of multiple focal therapies for prostate cancer, Dr. Jamil Syed recommends HIFU for certain patients with intermediate-risk prostate cancer, especially individuals with small, well-defined tumors localized to the lateral and posterior regions of the gland.