It’s the best path to definitive results, say leaders from Cleveland Clinic, Mayo Clinic

“The time has clearly come for a national coalition to coordinate hypothesis-driven clinical research trials to give the medical community the evidence it needs to safely and effectively treat and prevent COVID-19.” So argues Tom Mihaljevic, MD, CEO and President of Cleveland Clinic, in a new editorial he jointly authored with Gianrico Farrugia, MD, and Andrew Badley, MD, both of Mayo Clinic.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The editorial, published by STAT on Oct. 26, notes that while more than 2,000 interventional clinical trials for COVID-19 are listed on clinicaltrials.gov, many are investigating similar agents “and therefore competing for participants, infrastructure capacity, and funding.”
The three physician leaders advocate for a national, nonprofit clinical trials coalition to address COVID-19 by coordinating similar but disconnected studies for maximal returns and efficiency. The coalition should include experts in virology, immunology, public health, ethics, healthcare disparities, trial design and statistics, the editorial argues.
Functions of the proposed coalition would include (among others):
Citing their own organizations’ use of this type of coordinating group approach for research related to COVID-19, the three physicians contend that similar coordination at the national level will optimize resource use and help lead to “rigorous, generalizable, and definitive results.”
For the full editorial, click here.
Patients report improved sense of smell and taste
Clinicians who are accustomed to uncertainty can do well by patients
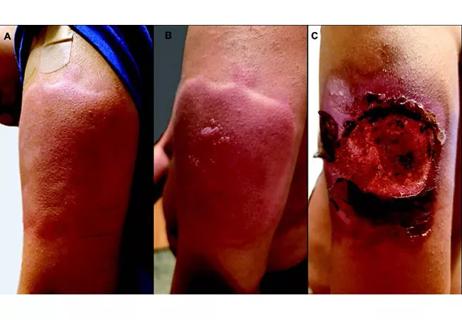
Unique skin changes can occur after infection or vaccine

Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition
As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients
Early results suggest positive outcomes from COVID-19 PrEP treatment
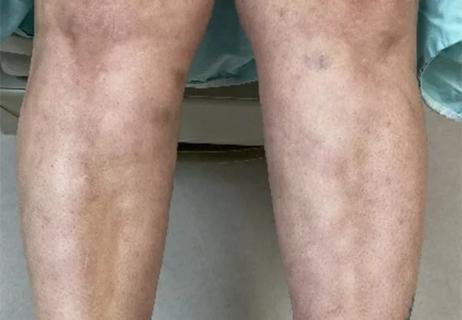
Could the virus have caused the condition or triggered previously undiagnosed disease?
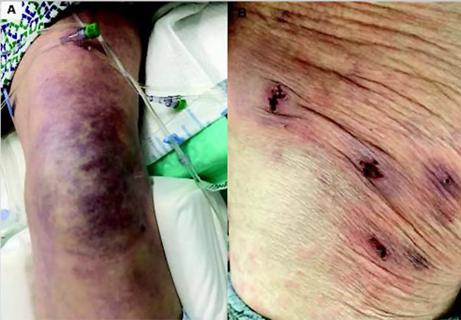
Five categories of cutaneous abnormalities are associated with COVID-19