Could the virus have caused the condition or triggered previously undiagnosed disease?
By Ambreesh Chawla, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Introduction
Eosinophilic fasciitis, also known as Shulman syndrome, is a rare autoimmune disorder that involves inflammation of the fascia overlying muscle, resulting in edema and deep induration of the extremities. It is typically symmetric and involves all four extremities. In contrast to scleroderma, the digits are typically spared and there is no association with Raynaud’s phenomenon or microscopic nailfold capillary changes. Eosinophilia is a characteristic laboratory finding in the early phase; however, it is not always present in early cases and is less prominent in later stages. Some cases of eosinophilic fasciitis can be associated with underlying hematologic disorders such as lymphoma, leukemia or aplastic anemia. Diagnosis is normally based on a deep excisional biopsy of the skin that includes the fascia.
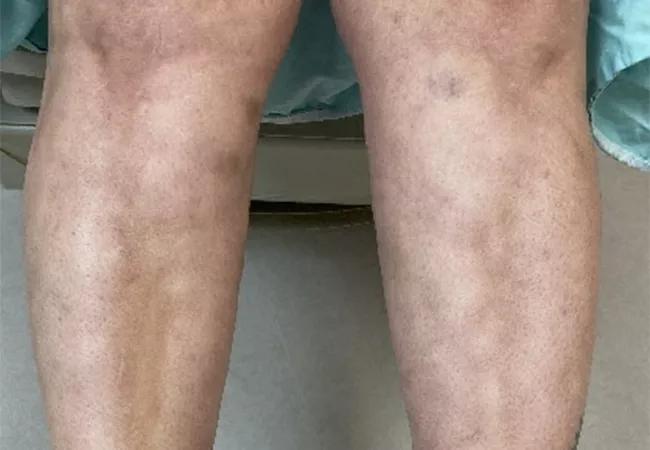
A 46-year-old female with a recent diagnosis of COVID-19 presented to Cleveland Clinic’s rheumatology clinic with new-onset worsening distal upper and lower extremity pain and discomfort. She noted that shortly after her diagnosis of COVID-19, she began to experience severe allodynia to light touch over her forearms and shins.
Laboratory testing revealed 25% eosinophils on complete blood count with mildly elevated markers of inflammation and borderline positive rheumatoid factor. Because of worsening allodynia, polyarthralgia and polymyalgia with persistent eosinophilia, the patient underwent an aggressive workup for eosinophilic-related conditions, including parasitic infections/strongyloides, vasculitis/eosinophilic granulomatosis with polyangiitis, blood disorders/hypereosinophilic syndrome, and allergy testing.
Advertisement
On a follow-up rheumatology evaluation, a physical exam revealed skin thickening and deep induration of her distal extremities (sparing her hands and feet) with a peau d’orange appearance of the skin of her forearms and shins. Magnetic resonance imaging revealed fascial thickening and edema predominantly involving the deep peripheral fascia superficial to the muscles in the forearms and lower legs. The patient subsequently underwent a full thickness biopsy, consisting of skin, fascia and superficial muscle, which demonstrated perivascular infiltration of histiocytes, eosinophils, lymphocytes and plasma cells. Based on these findings, she was diagnosed with eosinophilic fasciitis. The patient was then started on high doses of oral prednisone (1mg/kg/day) in addition to methotrexate. Treatment led to normalization of the eosinophil count and skin softening on subsequent evaluation.
While many autoimmune and rheumatologic conditions after viral infections have been described in the literature, to our knowledge this is the first case reported of eosinophilic fasciitis after primary COVID-19 infection. Development of new-onset autoimmune skin conditions has been reported following various vaccinations. Eosinophilic fasciitis has been documented after influenza vaccination and, more recently, has been described after a patient had received the COVID-19 mRNA vaccine. Other cutaneous disorders, such as morphea and discoid lupus, also have been described in the literature following various vaccinations.
Advertisement
The sequential association presented here between COVID-19 and newly diagnosed eosinophilic fasciitis could be coincidental. It is also possible that the COVID-19 primary infection triggered a phenotypic expression of a previously existing undiagnosed disease.
Patients with eosinophilic fasciitis typically respond with loosening of the skin to prolonged courses of high doses of steroids in conjunction with steroid-sparing agents such as methotrexate, mycophenolate mofetil and others. Treatment is typically required for several months to years. The condition overall caries a favorable prognosis, although joint contractures may persist if treatment is delayed.
Advertisement
Advertisement

Despite less overall volume loss than in MS and NMOSD, volumetric changes correlate with functional decline

It’s time to get familiar with this emerging demyelinating disorder

From dryness to diagnosis
Early experience with the agents confirms findings from clinical trials
Despite advancements, care for this rare autoimmune disease is too complex to go it alone
Treatment for scleroderma can sometimes cause esophageal symptoms
Patient’s symptoms included periorbital edema, fevers, weight loss
Good visual recovery and retinal reperfusion after prompt diagnosis and intensive therapy