Clinical trials for a urinary incontinence device are scheduled to begin soon
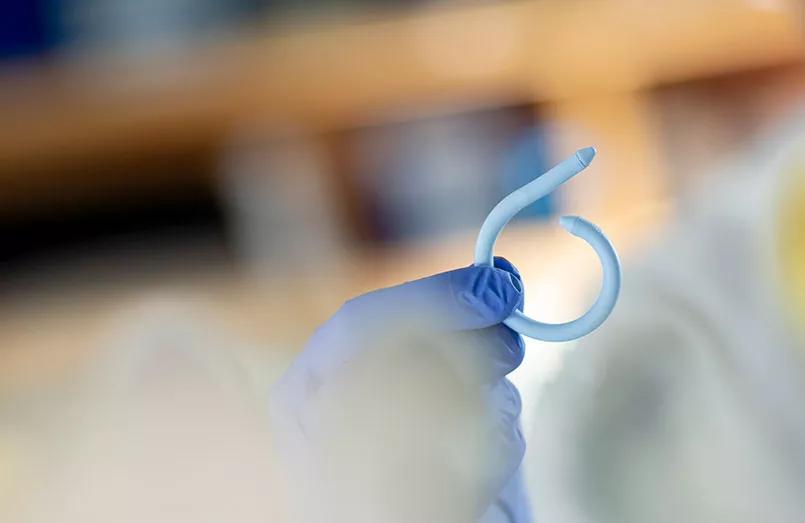
A wireless, insertable pressure sensor to assist in the diagnosis of urinary incontinence is now one step closer to clinical application in patients. The team is actively recruiting patients for a trial that will begin in early 2020.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Our vision is to take the monitoring out of the clinic, and take the catheter out of the monitoring,” says Margot Damaser, PhD, who created and oversees development of the UroMonitor.
Dr. Damaser, staff in the Biomedical Engineering Department of Cleveland Clinic’s Lerner Research Institute, likens the device to a “Fitbit for the bladder.” The flexible device, shaped like a coil, is inserted into a patient’s bladder lumen. It tracks physiological data over a four- to seven-day monitoring period and wirelessly transmits the information to the patient’s electronic medical record to inform a physician’s diagnosis and clinical management. The patient can then remove the device by a string and dispose of it completely.
Dr. Damaser works closely with urologists, including Howard Goldman, MD, staff in Cleveland Clinic’s Glickman Urological & Kidney Institute and clinical lead on the study, along with researchers and physician collaborators at Case Western Reserve University and the Louis Stokes Cleveland VA Medical Center.
Dr. Goldman notes that the short-term goal of the trial is to validate whether the UroMonitor demonstrates comparative clinical value to urodynamic testing with comparable or greater comfort. The team will insert the device in eight to 10 patients and monitor their activity in the clinic to determine the safety and efficacy of this diagnostic approach.
“This study will allow us to evaluate if the device provides, at a minimum, the same or similar effects as standard urodynamic testing,” he says. “That’s the first step.”
Advertisement
If the study shows improved outcomes for patients, the UroMonitor could represent a sea change in urodynamics.
Current diagnostic testing with a catheter is painful and embarrassing for some patients. After the catheter is inserted into patients’ bladders, they are required to force urination to simulate the issues they face during their daily activities. This simulation may also propagate artifactual data, notes Dr. Goldman, which is problematic for physicians.
The device would counter these issues. “It’s more comfortable for patients. A clinician inserts it, wirelessly turns it on and sends the patient home. For clinicians, there is more data to confirm the diagnosis and no need for specialized equipment or personnel to administer the test,” says Dr. Damaser.
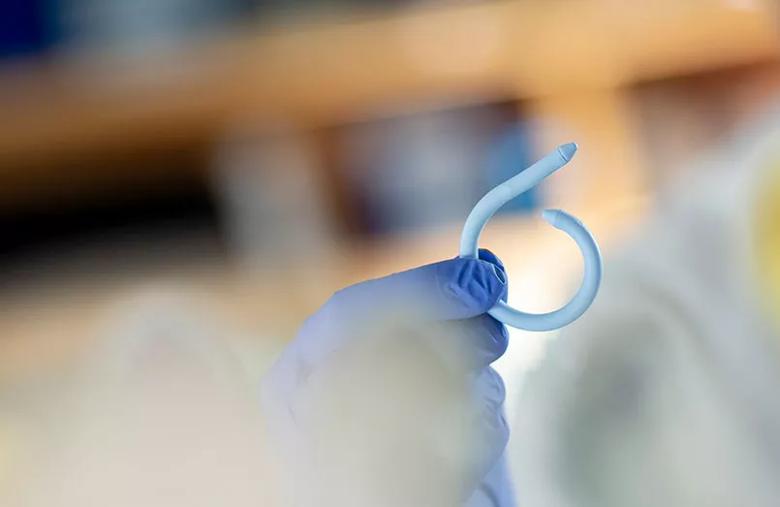
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6892c140-42ab-4adf-a5f9-b43f9cd7067e/805x-inset-Damaser_jpg)
Image of UroMonitor device
The device also has the potential to play a therapeutic role for patients with neurogenic etiologies. Possible applications include controlled drug delivery and feedback to neuromodulation or electrical stimulation systems. It may restore sensation for patients with pelvic floor dysfunction or inform them about when to empty their bladder.
On observing urodynamic testing 20 years ago, Dr. Damaser notes, “I thought, “Are you kidding? We can do so much better.” And that continues to be her mantra. With clinical trials set to begin in early 2020, she is already looking ahead to improve the device. She and her team are currently investigating volume sensing capabilities in the lab. “To fully realize a wireless, catheter-free ambulatory urodynamics device, we need to know how much is in the bladder — we need volume,” she says. “So that will be our next step.”
Advertisement
Advertisement

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Fixed-dose single-pill combinations and future therapies

Reproductive urologists publish a contemporary review to guide practice

Two recent cases show favorable pain and cosmesis outcomes

Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood

Proteinuria reduction remains the most important treatment target.

IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy

Oncologic and functional outcomes are promising, but selection is key