A brief review of expert guidance on what’s known and unknown

One of the leading COVID-19-related questions posed to Google in recent weeks concerns the issue of whether individuals can be reinfected with the virus after recovering from COVID-19. As experience shows, questions that start showing up on Google often soon start surfacing in medical appointments as well.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
While there’s still no clear answer to this question, practitioners do well to stay current on the issue so they can field patient questions most adroitly. To that end, a new review article in Cleveland Clinic Journal of Medicine’s COVID-19 Curbside Consults series can prove helpful. In the review, “COVID-19 Serologic Testing: FAQs and Caveats,” Cleveland Clinic immunopathologist Kamran Kadkhoda, PhD, addresses, among other issues, the question of whether the detectable presence of immunoglobulin G (IgG) antibodies — the antibodies largely responsible for long-term immunity after infection or vaccination — is reliable for evaluating infectivity and clinical immunity to COVID-19 reinfection.
“No one knows,” is the short answer from Dr. Kadkhoda, who then goes on to explain that patients with a positive IgG result on serologic testing may still be ill and can shed the SARS-CoV-2 virus through their respiratory secretions or stools. “Upper respiratory samples can remain positive for viral RNA for a few weeks after disease onset,” he notes, “when patients are supposed to have IgG antibodies.” Viral shedding in stool has been reported for up to 47 days,1 which Dr. Kadkhoda points out “speaks against authentic neutralizing capacity of tissue-transudated IgG and secretory IgA antibodies.” Moreover, the sister virus SARS-CoV has been grown in cultures from upper respiratory samples in 54% of cases at two weeks after symptom onset and in 16% of cases at three weeks after symptom onset — despite documented seroconversion in more than 92% of patients assessed by plaque-reduction neutralization test that detected “neutralizing antibodies.” 2
Advertisement
“Thus,” Dr. Kadkhoda writes, “having circulating neutralizing antibodies may not ensure lack of infectivity. This has yet to be shown in SARS-CoV-2.”
He adds that, as of early June 2020, the Centers for Disease Control and Prevention had not established guidelines for occupational health isolation disposition based on serologic testing, other than using two consecutive negative nucleic-acid amplification tests at least 24 hours apart.3
The review points out that the correlate of protection for COVID-19 is not yet known, although such correlates have been established for many other viral diseases and are sometimes routinely used for occupational health purposes, such as with hepatitis B. For COVID-19, the correlate of protection has to be established in large, well-designed randomized controlled trials, which have not been conducted. “Therefore,” Dr. Kadkhoda observes, “determination of ‘immune status’ of individuals, including healthcare workers, to SARS-CoV-2 cannot be established at this time using serology.”
The issue is further confounded, he adds, by the fact that individuals can be infected and become sick with common coronaviruses in the community in nearly every season and sometimes more than once within a season. “This suggests that immunity to some coronaviruses is short-lived, and lingering IgG antibodies from previous seasons does not mean an individual is necessarily immune to infection with the same coronaviruses.” Moreover, he notes, cell-mediated immunity (typically mediated through CD8+ memory T cells) also plays a role.
Advertisement
Dr. Kadhoda wraps up his response by noting that a recent report4 shows that 20% of COVID-19-infected individuals do not mount neutralizing antibodies and over 50% mount only low titer neutralizing antibodies with geometric titer of 142. While the remaining roughly 30% of individuals are able to mount high-titer neutralizing antibodies, “whether they will last and whether they are protective is not known,” he concludes.
For the full review by Dr. Kadkhoda, view the freely accessible COVID-19 Curbside Consult here.
Advertisement
Advertisement
Patients report improved sense of smell and taste
Clinicians who are accustomed to uncertainty can do well by patients

Unique skin changes can occur after infection or vaccine

Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition

As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients
Early results suggest positive outcomes from COVID-19 PrEP treatment
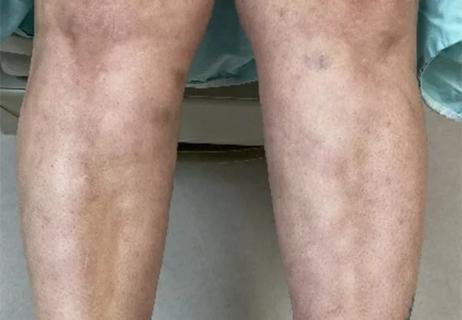
Could the virus have caused the condition or triggered previously undiagnosed disease?

Five categories of cutaneous abnormalities are associated with COVID-19