How iron and vitamin B6 influence the production of non-enzymatic H2S
In a study recently published in Nature Communications Biology, researchers from Lerner Research Institute identified a novel role of iron in the body, a discovery that may have important implications for diseases and disorders in which iron levels are affected, including sickle cell disease, hemochromatosis and select neurodegenerative diseases.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The team, led by Christopher Hine, PhD, Department of Cardiovascular & Metabolic Sciences, showed that iron, which is found abundantly in red blood cells, helps to catalyze a biochemical reaction in the body that produces hydrogen sulfide (H2S), an important bioactive signaling molecule that plays a role in many cellular processes.
There are two varieties of H2S—enzymatically and non-enzymatically produced. This study is an investigation into the latter, about which relatively little was previously known.
Enzymatic H2S is formed by the metabolism of sulfur-containing amino acids. Insufficient levels of this variety of H2S are associated with negative health outcomes, including the development of atherosclerosis, hypertension and select neurodegenerative disorders. In previously published work, Dr. Hine was the first to show that diet is a key regulator of enzymatic H2S production.
In the current study, Dr. Hine and his team describe for the first time how iron, together with vitamin B6, set into motion a chemical reaction that ultimately results in the production of non-enzymatic H2S. They show that vitamin B6 acts as a cofactor, meaning iron cannot jump start the reaction without it. In this way, levels of both molecules must be regulated for successful non-enzymatic H2S production.
The team went on to show that how much H2S is produced during this reaction is regulated by a few factors, including red blood cell and iron states.
They discovered that the iron found in damaged red blood cells has greater catalytic potential and produces more H2S than that of healthy, intact red blood cells. Damage can result from a variety of events and cellular processes, including lysis (or cell bursting) and protein degradation, as happens when bound iron uncouples to become free iron. Free iron, they found, produces more H2S than bound iron.
Advertisement
While this study uncovered much about the chemical reaction that produces non-enzymatic H2S and factors that affect its robustness, it is still unclear whether non-enzymatic H2S is protective, as is the case with enzymatic H2S, or detrimental. Dr. Hine and collaborators have already begun work on moving this next phase of investigation forward, as understanding how levels of iron-derived H2S relate to disease will have implications for maladies that affect iron homeostasis, hemorrhage and red blood cell integrity.
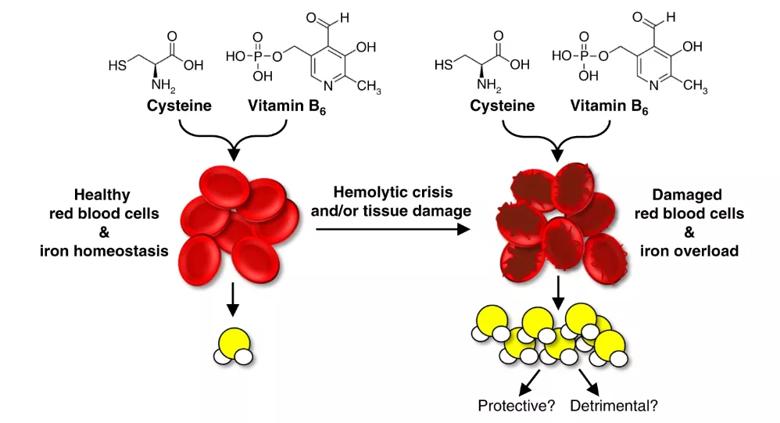
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/293d509d-a80b-4de9-9277-350a999b33d8/Hine-image-Nature-Comms-Biol_png)
Experimental model of red blood cell state and tissue integrity impacting iron-catalyzed non-enzymatic H2S production.
Jie Yang, PhD, a postdoctoral fellow in the Hine lab, is first author on the study. Other contributors from Cleveland Clinic include Paul Minkler, David Grove, PhD, Belinda Willard, PhD, and Raed Dweik, MD. The work was funded in part by a grant from the National Institute on Aging, part of the National Institutes of Health.
Note: This article was originally published by the Lerner Research Institute.
Images used under the Creative Commons license; originally published in: Yang J, Minkler P, Grove D, Wang R, Willard B, Dweik R, Hine C. Non-enzymtic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun Biol. 2019;2:194.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology