A practical review of pharmacology, potential indications, safety

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/16aa729c-12d9-402f-9fa1-cec961f8b5ea/antibiotics-and-myasthenia-gravis-exacerbations-1130531561)
Medications
By Joanne Schneider, DNP, RN, CNP; Mary Patterson, CNP;and Xavier F. Jimenez, MD, MA
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This article is reprinted from Cleveland Clinic Journal of Medicine (2019;86:807-814).
Most tricyclic antidepressants (TCAs) have FDA approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reup-take inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
Named for their chemical structure, TCAs contain three rings with one side chain. They are grouped into tertiary and secondary amine subtypes (Table 1).1
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Advertisement
Peak plasma concentration is at about two to six hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles and typical dosages for depression for commonly prescribed TCAs.
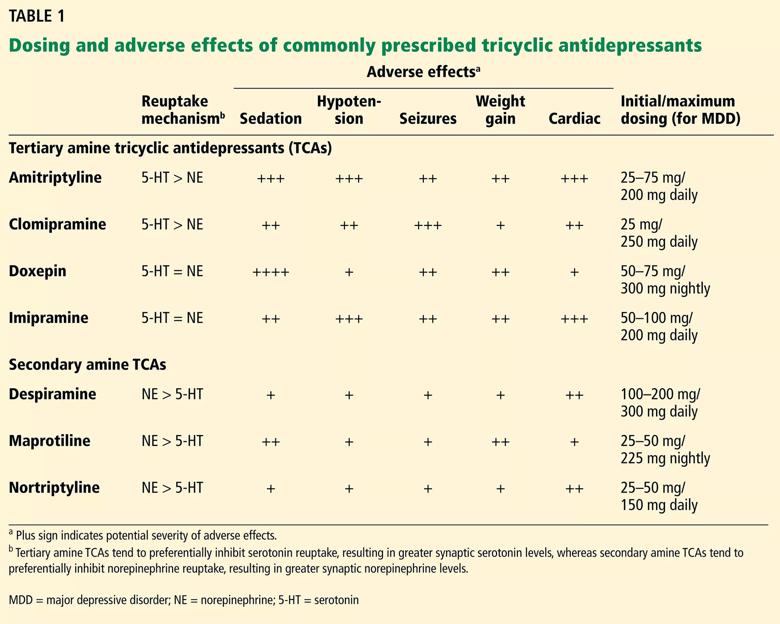
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b7707ca3-5416-4378-b9be-6fb86fbb726f/20-NEU-1863397-tricyclic-antidepressants-dosing-Table-1_jpg)
Headache and migraine. TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among various drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs — particularly amitriptyline — be reserved for patients who have both migraine and depression.
Advertisement
Neuropathic pain. Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 post-traumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25-150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain. Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily) and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis showed little evidence that TCAs were superior to placebo.19
Advertisement
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed six-month, double-blind, randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain. Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin) and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of nine placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine and milnacipran.24
Advertisement
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration and other modalities is strongly recommended.
Abdominal and gastrointestinal pain. TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms.Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and, to a lesser extent, nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after eight weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia. Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10-25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression.Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Tolerance to some effects may develop over time. If adverse effects prove to be a problem, therapy may need to be stopped or doses adjusted. Alternatively, adjunctive medications to address adverse effects may be considered (e.g., pilocarpine for dry mucous membranes, tamsulosin for urinary retention) (Table 2).
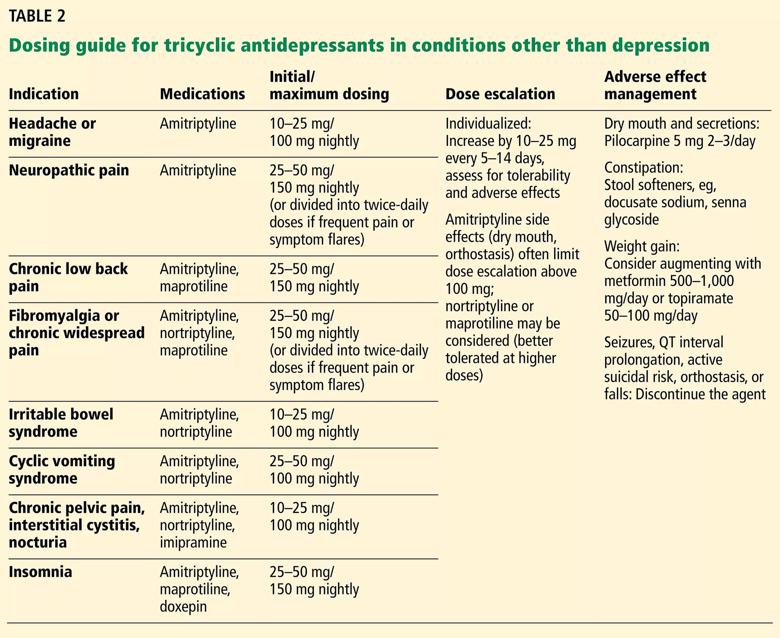
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ce1de229-4c72-41a8-9f61-f53c041622fe/20-NEU-1863397-tricyclic-antidepressants-dosing-Table-2_jpg)
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome. Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness and restlessness. Rebound depression, anxiety, panic or other psychiatric symptoms may also occur. Symptoms generally present within two to five days after dose discontinuation and last seven to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing, and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
Cardiac conduction abnormalities. TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (e.g., appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age. For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy. TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant MAO inhibitor treatment. Giving TCAs together with monoamine oxidase inhibitor (MAO) antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk. TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy. TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization and other intensive care.
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy and mental health optimization.
Ms. Schneider is with the Center for Comprehensive Pain Recovery in Cleveland Clinic’s Neurological Institute.
Advertisement
Ethical guidance provides guardrails so medical advances benefit patients
OCEANIC-STROKE results represent long-sought advance in secondary stroke prevention
Two studies from Cleveland Clinic may help advance the technology toward broader clinical use
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
An argument for clarifying the nomenclature
An expert talks through the benefits, limits and unresolved questions of an evolving technology
Recommendations on identifying and managing neurodevelopmental and related challenges
Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies