Obstructing key protein allows for increased treatment uptake for taxane chemotherapy
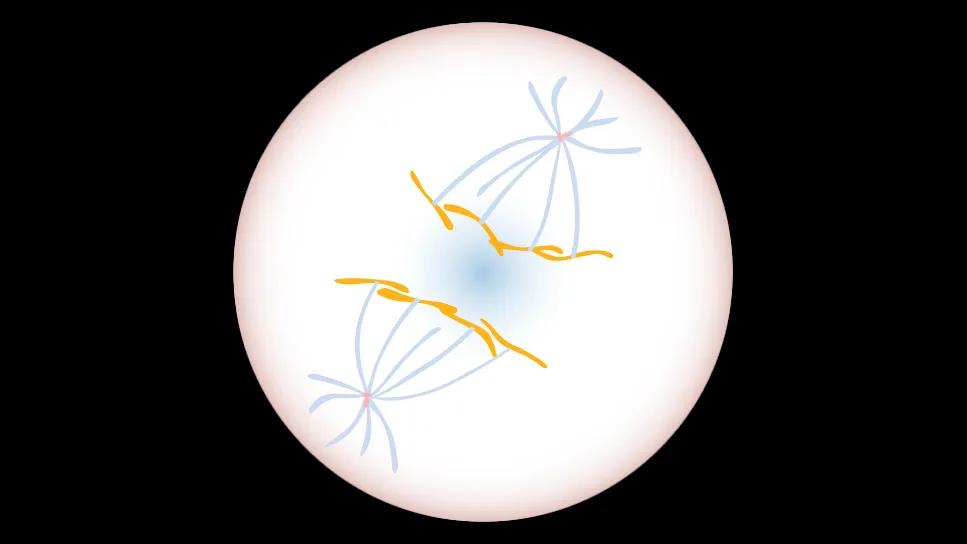
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/83ca9f08-aef2-4b92-83cb-0818859683a0/blocking-src-kinase-for-bc-1202981971)
DNA
DNA (orange) is reeled to opposite sides of a dividing cell by centrosomes (red and blue), to ensure the resulting daughter cells have the same genetic signature.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Researchers at Cleveland Clinic’s Learner Research Institute have determined the protein YES1 is a key contributor to poor outcomes and chemotherapy resistance in triple-negative breast cancer (TBNC). Blocking this protein creates vulnerabilities in drug-resistant TBNC cells, improving overall survival and reducing harmful side effects for a cancer with few available treatments.
The Cancer Research study was led by Ruth Keri, PhD, whose laboratory researches src kinases, a protein family known to promote cancer when mutated. Analyzing patient data revealed that the src kinase YES1 was highly elevated in TBNC tumors compared to other kinases. TBNC patients with higher YES1 levels in their tumors showed lower treatment responses and survival rates overall.
“Many cancer treatments target all src kinases indiscriminately, but some of these proteins act against each other so blocking them all isn’t always effective,” says Dr. Keri. “Now that we know YES1 is a key kinase driving triple-negative breast cancer, as well as how it works, we can begin to make more targeted treatments.”
Study co-first author Katrina Piemonte, PhD, an MD/PhD student who recently completed her thesis work in Dr. Keri’s lab, investigated YES1’s role in cell division for healthy and cancerous breast cells to determine how the protein drives TBNC. She found that YES1 is integral to making sure new cells have the correct amount of DNA after cell division.
For our body to make new cells, an established parent cell duplicates its genetic information and divides itself in half to create two new daughter cells. Before division, two molecules called centrosomes are created -- one on each end of the parent cell. The centrosomes act like fishing rods, reeling one copy of genetic material towards themselves at the edges of the cell. This process ensures each daughter cell receives a full set of genetic information. Having the right amount of DNA is necessary for the cancer cells to continue to grow.
Advertisement
Piemonte’s findings excited study co-author Natasha Ingles, another MD/PhD student in the Keri Lab. Ingles noted that taxanes like paclitaxel, which are the current standard of care treatment for TNBC, disrupt the same step of cell division as YES1 inhibitors by interacting with the “fishing line” that connects DNA to centrosomes.
Ingles tested combinations of taxanes with YES1 therapies in TBNC cells and preclinical models. She found that combination therapy was much more effective at eliminating TBNC cells than taxanes alone. Combining taxane chemotherapy with YES1 inhibitors increased taxane potency even at lower doses, which Ingles says helps reduce the risk of side effects. The team also showed healthy breast cells were virtually unaffected by YES1 inhibitors, further lowering the risk of potential side effects.
“As a physician-scientist in training, my goal is to improve clinical care through my research,” says Ingles. “If you get breast cancer chemotherapy, you're most likely getting taxanes. Our research shows that blocking YES1 makes triple-negative breast cancer more vulnerable to taxanes. My hope is that these findings pave the way forward to increasing the treatment’s effectiveness, while reducing the risk of negative side effects.”
Dr. Keri hopes to conduct clinical trials for YES1-taxane combination therapies soon. Her team is also testing YES1’s ability to combine with other forms of chemotherapy to improve cancer outcomes.
Note: This is an abridged version of an article originally published by Cleveland Clinic Learner Research Institute.
Advertisement
Advertisement
Real-world results reporting aims to make treatments safer and more effective
Polygenic risk score could help predict who will develop this aggressive breast cancer
Combination of olaparib and carboplatin results in complete durable response for a patient with BRCA2 and “BRCAness” mutations
Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer
Reconsidering axillary lymph node dissection as well as depth of surgical margins
Ultra-Hypofractionated Whole Breast Irradiation and Partial Breast Irradiation Reduce Many Toxicities
Robotic-Assisted Procedures Offer Breakthroughs in Lymphedema Treatment After Breast Cancer Surgery
Best practices for reducing toxicities