Will new or improved modalities help patients accept more invasive treatment?
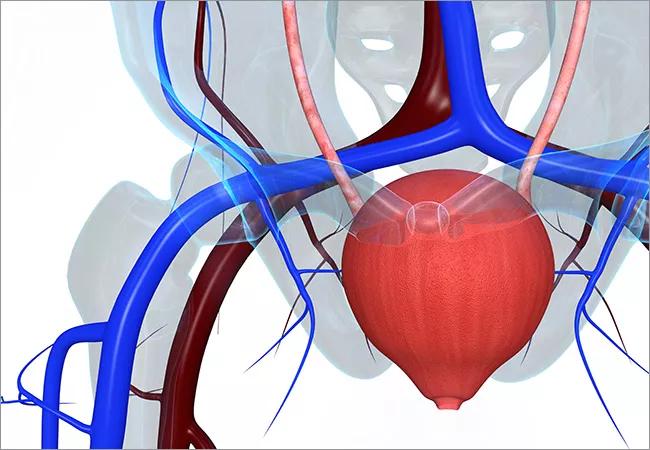
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/12d3293c-7d07-43ac-9a81-5e8825a80c69/650x450-Overactive-Bladder-Goldman_jpg)
650×450-Overactive-Bladder-Goldman
Overactive bladder (OAB) affects more than 40 million Americans, and can seriously diminish quality of life. Treatments range from simple to complex, depending on the severity of patients’ symptoms and their tolerance for medication side effects or invasive procedures.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
First-line therapies for OAB include lifestyle modifications and physical therapy in the form of pelvic floor exercises. Second-line therapies involve medications such as antimuscarinic drugs and a beta-adrenergic receptor agonists. However, these have a high failure rate, with compliance at only 25 percent after one year. Third-line therapies — some of which are interesting innovations — include Botox injections and tibial or sacral nerve stimulation plus a new device that uses radiofrequency technology.
Cleveland Clinic physicians recently published a review article in the Current Urology Reports that evaluates new OAB devices and technologies.
“Some of these new technologies are simply improvements on methods already in use for controlling OAB symptoms, while others offer a whole new way of attacking the problem,” reports urologist Howard Goldman, MD, one of the study co-authors. Their review is summarized below.
Sacral nerve stimulation (SNS) has been used to treat OAB for several decades. The SNS device is implanted above the buttocks. A lead sends electrical impulses to the sacral nerves to calm the bladder (Figure 1).
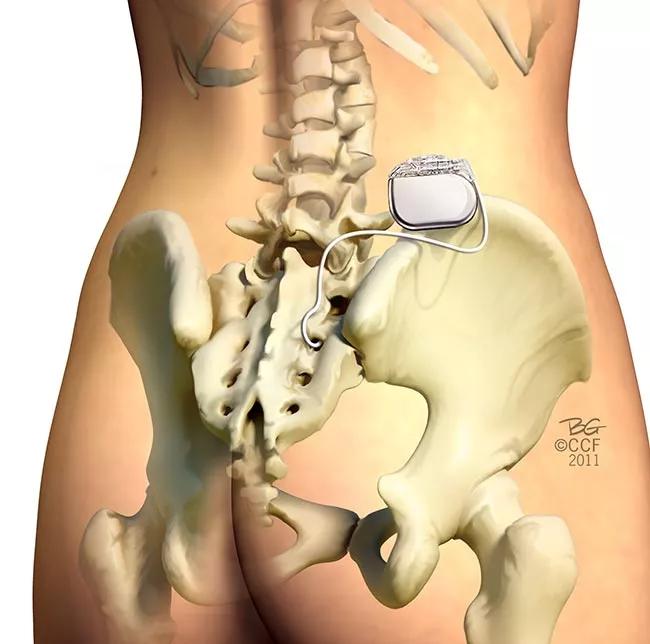
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b10f7049-8abf-44e4-b556-4041f0b600d1/Inset-2_jpg)
Sacral nerve stimulation for OAB.
Only one SNS device is FDA-approved and on the market — Medtronic’s InterstimTM. Studies show that 83 percent of OAB patients who use the device experience a significant reduction in symptoms (an average of 2.3 fewer leaks per day or 5 fewer voids). The downside of this technology is the potential need for battery replacement as early as three years after implantation. Also patients generally cannot undergo non-head MRIs because of potential damage to the device and injury to the patient.
Advertisement
Axonics Modulation Technologies, Inc. has developed an SNS device that is 60 percent smaller than the Interstim — about the size of two quarters. With its rechargeable, implantable pulse-generator battery, it has an expected 15-year life in the body. It is current-controlled so that output voltage is automatically adjusted based on tissue impedance, which may provide more consistent therapy, Dr. Goldman explains. The company hopes the device will prove safe for certain types of MRI procedures.
Three-month data from an ongoing European prospective multicenter trial of this device demonstrate a 73 percent response rate with more than 50 percent improvement in OAB symptoms, Dr. Goldman reports. Eighteen percent of subjects reported device-related adverse events, but no charging-related adverse events were reported. A large U.S. multicenter study, of which Dr. Goldman is the primary investigator, has recently begun and early results are expected within six months.
Another OAB treatment modality included in the recent review is percutaneous tibial nerve stimulation (TNS). Physicians insert a small needle into the ankle to stimulate the tibial nerve once a week for the first three months, followed by ongoing monthly appointments (Figure 2).
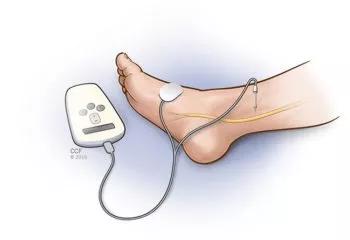
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7c6026e6-dec1-413a-85cc-2557336e773c/Inset-1_jpg)
Tibial nerve stimulation for OAB.
Two new implantable TNS devices have potential to eliminate the need for the frequent office visits. One is the Bioness StimRouterTM neuromodulation system. It has an implanted lead with an integrated receiver, anchor and three electrode contacts in close association to the posterior tibial nerve. The lead captures stimulation energy that is delivered wirelessly and transdermally from a rechargeable external pulse transmitter and electrode patch. Both the transmitter and patch are worn only during periods of stimulation. The patient uses a programmer to track usage and change programs. The programmer controls the external pulse transmitter wirelessly. Dr. Goldman is the site principal investigator for an ongoing clinical trial of this technology.
Advertisement
BlueWind Medical’s RENOVATM TNS device can be surgically implanted or percutaneously injected in close vicinity to the tibial neurovascular bundle. Powered through wireless technology, this device includes an external control unit worn around the ankle during treatment — 30 minutes daily. Using a computer program, the physician sets stimulation parameters for each patient to optimize therapeutic outcome.
Clinical trial results for this device showed 71 percent of subjects experienced greater than 50 percent improvement in OAB symptoms at the six-month follow-up, with 27.6 percent of urgency incontinence subjects being dry at the six-month follow-up, Dr. Goldman’s group reported in their review.
Abnormal electrical activity may start in one area of the bladder then spread across the organ, causing OAB symptoms, Dr. Goldman explains. Two companies are developing technologies designed to disrupt or contain that activity.
NewUro’s transurethral bladder partitioning (TBP) device uses controlled radiofrequency energy to ablate thin tissue lines to fence off abnormal electrical activity in the bladder. NewUro’s TBP has a disposable radiofrequency catheter that is inserted through the urethra and expanded like a balloon so it is in contact with the bladder wall. A radiofrequency generator sends power to the catheter, creating thin radiofrequency ablation lines that partition the bladder into electrically independent zones. This device has not yet undergone human clinical trials.
Amphora Medical is developing a cystoscopic device capable of ablating areas near the trigone — an area in the bladder where it is thought many of the nerves that control function pass through — with radiofrequency energy. This could dampen nerve signals and restore normal bladder function. The company is currently running two clinical trials, one in Belgium and Canada and one in the U.S.
Advertisement
With these innovations and inventions on the horizon, patients will soon have more and better choices for coping with their OAB symptoms, Dr. Goldman says. “The hope is that these devices will expand patient acceptance of OAB treatments.”
Once such devices are available, he suggests that urologists gauge their patients’ personal preferences when choosing one. “It depends on the individual,” he says. “Some patients may not want a device implanted in their buttock, while others may not want to have to wear something on their ankle at night for half an hour.”
More from Cleveland Clinic about overactive bladder.
Dr. Goldman is a consultant for Medtronic, Nuvectra, Axonic and NewUro, a study participant for Bioness, and receives study support from Medtronic.
Advertisement
Advertisement
Pediatric urologists lead quality improvement initiative, author systemwide guideline
Fixed-dose single-pill combinations and future therapies
Reproductive urologists publish a contemporary review to guide practice
Two recent cases show favorable pain and cosmesis outcomes
Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood
Proteinuria reduction remains the most important treatment target.
IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy
Oncologic and functional outcomes are promising, but selection is key