A new discovery may help slow the growth of tumors — and change the research and treatment of cancer.
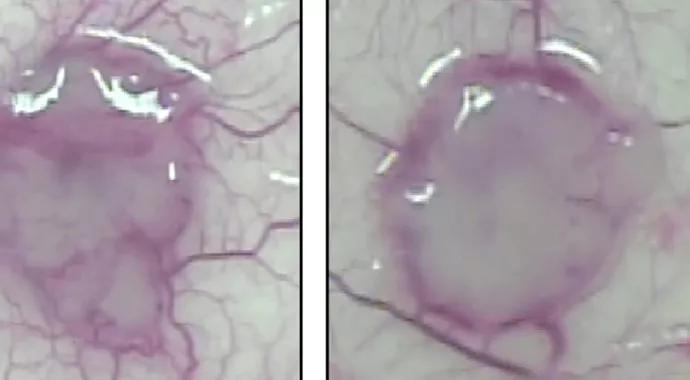
The vast majority of cancer-related deaths are attributed to metastasis, which makes it a critical target in the ongoing study and treatment of cancer.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The ability to slow — or prevent — tumors from growing and spreading could revolutionize oncological intervention and outcomes. So recent progress by Cleveland Clinic researcher Paul Fox, PhD, is attracting attention. The discovery of a new variation in a known family of proteins affirms the old adage that sometimes big things come in small packages.
Dr. Fox’s work relates to vascular endothelial growth factor A (VEGF-A), which is vital for the development of blood vessels and the proliferation of endothelial cells. It also plays a part in wound healing, inflammation and female reproduction and menstruation.
But there is a cruel irony behind this important protein: VEGF-A has long been known to stimulate angiogenesis in solid tumors, increasing the likelihood of growth and metastasis. Scientists worldwide have examined whether blocking VEGF-A can serve as a therapeutic tool against cancer. In fact, such therapies currently exist to treat colon and kidney cancers, and researchers are investigating them for other neoplasms.
A team led by Dr. Fox, of Cleveland Clinic’s Lerner Research Institute, has uncovered a variant of VEGF-A that might provide additional guidance for developing novel and effective cancer treatments.
The new protein was generated when a stop codon — which instructs the gene to terminate the translation of its genetic code, much like a period at the end of a sentence — was ignored. This process, known as a programmed translational read-through, transformed VEGF-A into a slightly altered variant, which Dr. Fox and his colleagues have named VEGF-Ax (“x” is for extended).
Advertisement
This tiny transformation causes a not-so-tiny effect: It appears to reverse the behavior of its parental form, VEGF-A. That means that it halts rather than promotes angiogenesis and tumor growth.
“It is truly remarkable that a small addition in a protein sequence leads not just to a protein with a different function, but one with a function completely opposite to the original,” explains Dr. Fox. “In the context of cancer, the small extension changes a very ‘bad’ protein into a very ‘good’ one.”
The initial discovery of VEGF-Ax occurred in animal models, but it may prove relevant to humans through future studies. The end goal could be an injectable VEGF-Ax to slow tumor growth as a direct therapy.
Dr. Fox notes some potential indirect benefits as well, such as its utility as a biomarker to inform treatment decisions. Specifically, conventional anti-VEGF-A therapy would not only inhibit its tumor-stimulating properties, it would unfortunately also block the beneficial, anti-angiogenic effects of VEGF-Ax. Thus, if a tumor is producing a large amount of VEGF-Ax, clinicians might want to avoid such a treatment.
“Similarly, this is also instructive to clinicians right now who are researching VEGF therapy and may want to start thinking about therapies that will block regular VEGF-A, but not this new extended form,” adds Dr. Fox.
The next stage in research will focus on expanding findings from animal studies. For instance, it is unclear whether toxicity is an issue. Additional data on dosage and frequency of administration will be imperative. But despite these and other pending questions, Dr. Fox remains cautiously optimistic about the implications of the discovery.
Advertisement
“Is this likely to lead to a cure of cancer? Who knows,” he says. “Is it likely to improve the therapies being used against cancer? I think there’s a pretty good chance that this will be an important contribution to our understanding of how to apply anti-angiogenic therapy.”
Advertisement
Advertisement

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units