
Screen patients seeking care for chlamydia, gonorrhea
Research finds low rates of positive test results
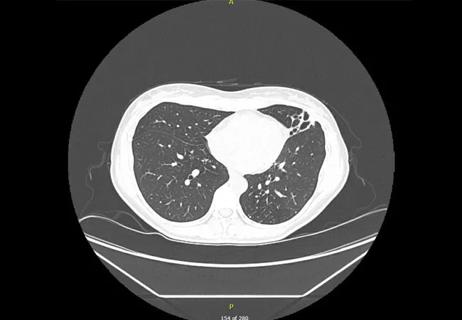
Lingulectomy removes infection when antibiotics fail

Researchers have developed immunoprofiles for an emerging disease with a mortality rate as high as 27%
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy

A brief review of expert guidance on what’s known and unknown

Screening assay effective across all stages, can identify tissue of origin
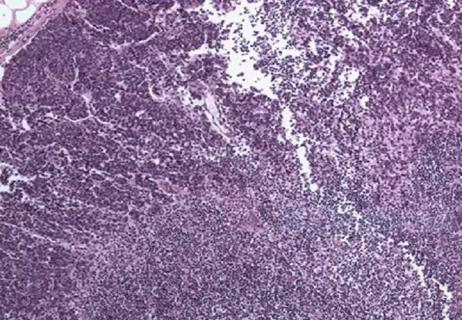
Leveraging the prognostic significance of sentinel lymph node biopsy histologic patterns
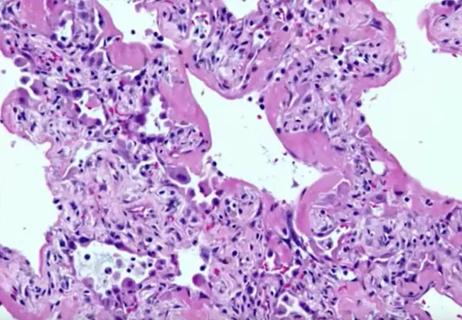
ARDS, diffuse alveolar damage and hypoxia
Molecular study suggests an update to AJCC guidelines

Findings from one of the first published case series
Advertisement
Advertisement