Patient achieves complete remission from aggressive marginal zone lymphoma with liso-cel
In March 2021, a patient in her early 50s with pelvic pain was referred to Cleveland Clinic Cancer Institute due to suspicion of lymphoma. After receiving a complete workup, followed by CAR T-cell therapy, she achieved a full response to treatment.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The patient had an ovarian mass that her OB/GYN had followed for years but when her lymph nodes became enlarged, he ordered a biopsy of the lymph nodes, which was not definitive. Since there are many types of B-cell lymphoma with varying treatment modalities, it was important to determine the exact histology.
Originally from Puerto Rico, the patient spoke very little English, so the clinical team spoke with her through an interpreter phone. “The first challenge was explaining that that we needed to do additional testing,” recalls Allison Winter, MD, the patient’s hematologist.
Interventional radiology sampled a lymph node near the retroperitoneum, but it was too small to be diagnostic, so the patient returned for a second sample. The follow-up biopsy identified marginal zone lymphoma, which is a low-grade B-cell non-Hodgkin’s lymphoma. Her clinical course, however, did not follow the typical low-grade lymphoma.
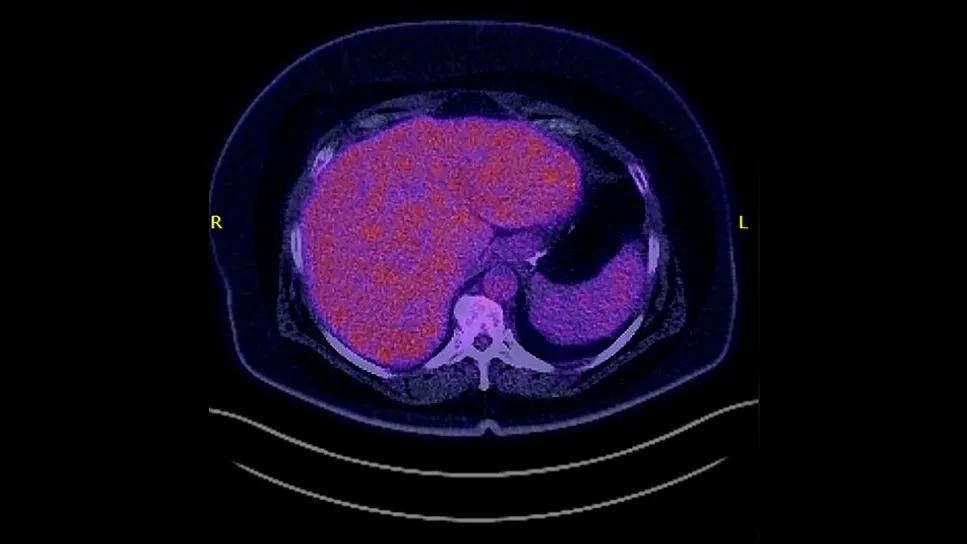
Dr. Winter ordered a PET scan, which revealed high standardized uptake value (SUV), a gauge of the level of metabolic activity. The SUV of 23 was indicative of more aggressive disease. “I explained that the growth in her pelvis appeared to be benign, but it was the lymph nodes we were concerned about. Even though the biopsy showed low-grade lymphoma, she would need more aggressive chemotherapy since the PET scan showed evidence that there was a concern for a higher-grade process. That was a tough pill for her to swallow that she would need aggressive chemotherapy and also lose her hair.”
Advertisement
The patient completed six cycles of rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate and prednisone (R-CHOP). A PET scan showed a complete remission by that September. Six months later, a surveillance CAT scan found some growth of lymph nodes as well as enterocolitis in her colon, which initially appeared to be an infection. Unfortunately, a repeat CAT scan two months later revealed that although the infection in her colon was gone, the lymph nodes were larger.
“Progression within the first 24 months after treatment with R-CHOP is a very negative prognostic sign in other forms of lymphoma,” explains Dr. Winter. She prescribed the oral BTK inhibitor zanubrutinib, which is approved to treat marginal zone lymphoma. However, the disease did not respond to the therapy.
In similar cases with follicular lymphoma that returns after R-CHOP, clinicians recommend novel agents, stem cell transplant or CAR T-cell therapy. Marginal zone lymphoma has a separate histology, though, and there is still no FDA-approved CAR T-cell therapy for it. “Since she had zero response to zanubrutinib, I knew we were in trouble and needed CAR T-cell therapy,” recalls Dr. Winter.
Dr. Winter explained to the patient that CAR T-cell therapy wasn’t FDA-approved for marginal lymphoma since there are so few patients with this specific histology. The only way to give her the therapy would be through a clinical trial using a commercially available CAR T-cell treatment for other B-cell lymphomas. Initially, the patient was very apprehensive and told the research nurse she wasn’t interested.
Advertisement
“I knew in my heart this was the best choice. I wasn't going to be able to sleep at night until I knew that she understood her options,” says Dr. Winter. Meeting with the patient and her husband, Dr. Winter started out by asking open-ended questions to understand what her patient’s apprehension was. Gleaning these insights helped guide the discussion in a positive way.
“Like a lot of people, her initial concern was that she thought a clinical trial meant it was first-in-human testing,” she says. “Patients often think they’re going to be a Guinea pig. They don’t understand that there are a lot of different types of clinical trials. I explained that this therapy works well for a similar disease and the way we bring this therapy to patients like her is to safely monitor them through a clinical trial.”
Since patients also need caregiver support for many weeks after CAR T-cell therapy, it was also crucial to work out those logistics in advance. The patient’s husband was very supportive but had a busy work schedule. The care team talked with the family about what support she would need as well as guidance in navigating FMLA. They also offered to speak with the patient’s brother, who is a nurse. The patient had a supportive family, who rallied around her and her husband throughout the treatment.
After going through an initial screening process, the patient was enrolled in a clinical trial using liso-cel CAR T-cell therapy for patients with marginal zone lymphoma. Throughout the process, Dr. Winter sought out additional opportunities to talk with her patient in person to build a connection, including checking in on her during the apheresis to collect her T-cells. She received some radiation prior to her CAR T-cell procedure to reduce the tumor burden and hopefully minimize side effects.
Advertisement
In March 2023, the patient was admitted to the hospital for infusion of the CAR T-cells and monitoring for side effects. She had no adverse events such as cytokine release syndrome or neurotoxicity, and was discharged after a seven-day stay.
The patient achieved a complete response to the CAR T-cell therapy. More than 2.5 years later, she remains in full remission. She’s exercising at the gym and is feeling well.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/387e87d2-3ceb-4bc9-883a-f61973b30d32/car-t-cell-therapy-marginal-lymphoma-inset)
Looking back on the experience, Dr. Winter shared several takeaways.
Face-to-face interactions make a difference. “When delivering difficult news, you need to be incredibly patient. As physicians, we tend to use language that is far more complicated than most people understand. The only way patients are going to understand the ‘why” behind what we’re recommending is if they trust us. Building trust requires investing the time to explain the situation at a level patients understand.”
Respect patients’ autonomy. “Respect patients’ opinions about what they want to do with their own bodies,” she advises. “Be certain that they understand the rationale behind what you’re recommending, so you know that their choices lines up with sound medical decision-making. But it’s just as important to really listen to what they’re saying.”
Use visual aids. Dr. Winter brings her laptop and shows pictures during patient discussions as an additional educational tool. Additionally, to reduce communication barriers, she now has a research coordinator on her team who speaks Spanish.
Be proactive. Consider the situation ahead of time and identify patients who may need more of your time. In this case, that involved meeting with the family to explain the reason behind recommending CAR T-cell therapy, dispel myths about clinical trials and discuss ways the patient’s extended family could support her during and after treatment.
Advertisement
Address patients’ concerns about weight. “We use a lot of steroids in lymphoma treatment, which can cause weight gain. The patient had had a laparoscopic sleeve gastrectomy in 2020, so was understandably upset when she regained weight during lymphoma treatment. I always tell my patients in these situations that they are beautiful inside and out and this is temporary. On my patient’s most recent visit, I was so proud of her for getting back into good habits and exercising. I commended her for losing weight in a healthy way.”
Advertisement

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists