Patient doing well, stimulation to start in coming weeks
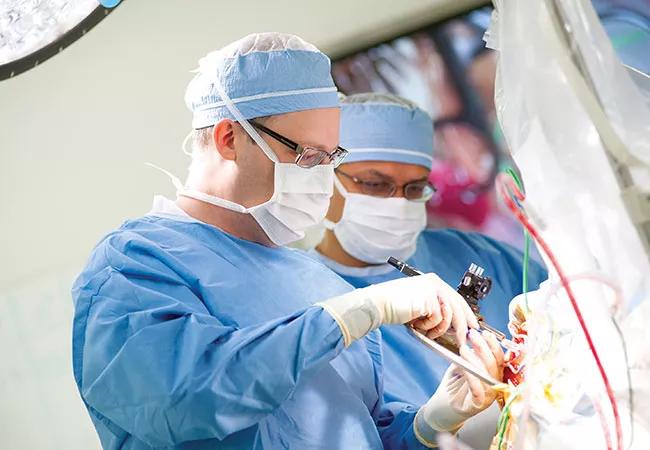
Cleveland Clinic has performed the first deep brain stimulation (DBS) surgery in a patient for the purpose of restoring motor function following ischemic stroke.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
During a six-hour procedure on Dec. 19, 2016, a team led by Andre Machado, MD, PhD, implanted DBS electrodes in the cerebellum of the patient, who has severe post-stroke residual hemiparesis despite previous physical therapy. The patient has been discharged home feeling well and in stable condition.
Over the next few weeks, following adequate healing and recovery from the brain surgery, the patient will undergo physical therapy. After a few weeks of rehabilitation, the DBS device connected to the implanted electrodes will be turned on as the patient continues physical therapy. The patient will be monitored and evaluated regularly to determine whether and how DBS can enhance the effects of physical therapy.
The first-in-human operation is the initial case in an NIH-supported Cleveland Clinic trial of DBS for stroke recovery detailed previously on Consult QD.
“If this research succeeds, it is a new hope for patients who have remained paralyzed after a stroke,” says Dr. Machado, Chairman of Cleveland Clinic’s Neurological Institute and a neurosurgeon specializing in neuromodulation. “It is an opportunity to allow our patients to rehabilitate and gain function and independence. Our knowledge to date shows that deep brain stimulation can help the brain reorganize and adapt beyond what physical therapy alone can do. Our study’s goal is to boost rehabilitation outcomes beyond what physical therapy alone could achieve.”
More details about this initial case in the trial are available in a new Time magazine feature. Dr. Machado (shown in the foreground of the above photo) discusses the trial’s rationale and goals in this short Consult QD video.
Advertisement
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help