Also will assess ability of high-intensity exercise to offset the cognitive decline
A clinical trial planned by Cleveland Clinic researchers aims to identify the neural signature underlying cognitive dysfunction in patients with Parkinson’s disease (PD) and determine if high-intensity exercise — which has been shown to improve motor functioning — can mitigate PD-associated cognitive decline.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
If successful, the ambitious project could improve and help personalize the treatment of PD, according to principal investigator Jay Alberts, PhD, Vice Chair of Innovation in Cleveland Clinic’s Neurological Institute and a staff member in the Department of Biomedical Engineering.
Potential benefits include gaining a better understanding of the neural mechanisms that underlie cognitive function in patients with PD; developing an evidence-based therapeutic aerobic exercise protocol; refining deep brain stimulation (DBS) programming to minimize cognitive impairment; and possibly using DBS to replicate the neural effects of exercise.
“The precise effects of exercise on PD patients’ cognitive function and their ability to perform dual tasks has not been systematically evaluated,” Dr. Alberts says. “This leaves a major gap in the potential optimization of exercise to address the cognitive declines associated with PD. With our results, I hope we can close that gap.”
The research is supported by a new three-year, $1.2 million grant from the U.S. Department of Defense. It builds on years of prior work by Dr. Alberts and colleagues to explore aerobic exercise’s disease-altering potential in PD. That research has shown exercise can change motor connectivity and improve information processing and motor performance, mobility, bimanual coordination and olfaction.
Dr. Alberts is a principal investigator in two current clinical trials, SPARX3 and CYCLE-II, that also are evaluating whether long-term high-intensity exercise programs can alter PD progression. The planned trial will delve deeper, using new technology to probe aerobic exercise’s impact on neural activity in patients with PD.
Advertisement
While research using animal models of PD and in human participants has demonstrated that aerobic exercise improves central nervous system functioning, its precise means of achieving this outcome and whether there is an associated gain in cognitive function and task performance capabilities in PD has not been systematically studied.
PD is characterized by excessive synchronous neural activity in the beta frequency (14–30 Hz) across the basal ganglia-thalamic-cortical motor network, specifically in the subthalamic nucleus (STN). The STN occupies a zone of important cortical input and plays a major role in locomotion, balance and motor coordination. There is also evidence of altered cortical theta frequency (4-7 Hz) oscillations in PD, which correlates with cognitive decline.
Dr. Alberts and his team believe that high-intensity exercise, especially forced exercise — where a patient’s pace is mechanically accelerated, as opposed to unassisted voluntary exercise — reduces STN beta hypersynchrony and theta activity, improving functionality of the cortico-basal ganglia-thalamocortical circuit and, in turn, cognitive functioning.
“We think forced exercise is better than voluntary exercise because you have a higher amount of afferent information — both the quantity and quality of information — going back to the brain,” Dr. Alberts says. “That would then trigger either a change in neurotrophic factors or dopamine or network behavior in cortical regions. While we expect improvements in performance and attenuation in beta-band activity following a single bout of both voluntary and forced exercise, we think forced exercise will result in superior outcomes in cognitive and dual-task performance because of greater beta and theta attenuation.”
Advertisement
Until recently, testing the neural signaling aspect of that exercise-impact hypothesis was not possible. Recording neural activity in the STN could only be done during DBS surgery or when the electrode was temporarily externalized immediately after surgery.
In 2020 the FDA approved a new DBS device that, in addition to delivering therapeutic stimulation, can bilaterally detect and record neural signals in the STN in everyday situations. The research and clinical applications of this technology are only beginning to be explored.
In the upcoming clinical trial, Dr. Alberts and his team plan to simultaneously record neural activity from the STN using the new DBS system and cortical activity using EEG in PD patients. The recordings will take place before, during and after forced and voluntary exercise, and while the patients perform cognitive and dual-task activities.
“We’re not measuring neurotrophic factors, but with STN recording we can readily determine if there are differences in how these two types of exercise impact the PD neural network,” Dr. Alberts says. “The cleanliness of the signal and the quality of data are unprecedented.”
The clinical trial will enroll 25 patients with PD who have previously undergone DBS surgery utilizing the recording DBS system. Data gathering will occur while the patients are temporarily off their PD medication and without DBS stimulation. The participants will undertake both voluntary and forced exercise on a stationary cycle.
DBS and EEG recording during the 150-minute experiment session will occur in six phases: with patients at rest; while patients at rest undergo cognitive and dual-task testing; while patients undergo voluntary and forced exercise; while patients undergo cognitive and dual-task testing after exercise; while patients are at rest after exercise; and while patients undergo a second round of post-exercise cognitive and dual-task testing.
Advertisement
Cognitive assessments will include Reaction Time, the Trail Making Test parts A & B, and the n-back test. An upper-extremity dual-task challenge (force tracking + n-back) will allow the researchers to evaluate patients’ motor/cognitive interaction.
The data resulting from DBS and EEG recording during the at-rest, exercise and testing phases should provide a neural signature representing PD-related cognitive decline and the effects of exercise. “We’ll be able to see what’s happening in the STN and how that impacts the cortical regions such as the dorsolateral prefrontal cortex and the supplementary motor area,” Dr. Alberts says. The researchers will look specifically for differences in neural activity and synchrony in the beta and theta frequencies.
Identifying the neural signature of PD cognitive dysfunction — a finding that would need to be validated with larger studies — would set the stage for improved or new therapies, Dr. Alberts says, including personalized approaches intended to mitigate decline.
Initially, that could manifest as an evidence-based exercise program for PD patients. “What I hope is that we’ll have something that will help us understand what potential might exist for exercise in more advanced patients,” Dr. Alberts says. “And that clinicians and providers will view exercise as medicine and think about it in a very prescriptive way, based on a patient’s neural patterns, rather than simply saying they should walk or move around more.”
Longer-term, finding a neural signature associated with cognitive dysfunction could help neurosurgeons and neurologists refine DBS electrode placement and stimulation programming, and possibly justify supplementing stimulation with exercise, Dr. Alberts believes.
Advertisement
Although DBS is effective at improving the motor symptoms of PD, its protracted use is associated with declines in cognitive performance, especially executive functions such as verbal fluency, compared with the cognitive status of PD patients who have not undergone DBS implantation.
“The subthalamic nucleus has three subdivisions: sensorimotor, limbic and associative,” Dr. Alberts explains. “With a DBS electrode in the STN, you’re activating a large volume of tissue. If you have a spread of current from the sensory-motor region to those more cognitive areas — limbic and associative — you are most likely disrupting normal cognitive function.”
A better understanding of the neural mechanism of PD cognitive dysfunction “could help in terms of electrode location,” Dr. Alberts says. “Maybe you could turn down voltage or amplitude or direct the current more to the sensorimotor area. Also, maybe we can use exercise to supplement DBS.” By exercising in tandem with stimulation, patients “could still get motor benefit while potentially preventing cognitive losses.”
Lastly, Dr. Alberts believes, a neural signature of exercise’s cognitive benefit could provide the basis to investigate the use of DBS to replicate the neural effects of exercise.
“We’re not suggesting DBS would make exercise obsolete,” he says. “But for patients who are not able to exercise for various reasons, replicating the neural effects of exercise via DBS may provide benefits that would normally be unrealized due to mobility issues. That’s far in the future, but it’s not beyond the realm of possibility.”
Advertisement

Various AR approaches affect symptom frequency and duration differently

Dopamine agonist performs in patients with early stage and advanced disease

Early assessment could affect clinical decision making

Systems genetics approach sets stage for lab testing of simvastatin and other candidate drugs

Study aims to inform an enhanced approach to exercise as medicine

New tool for general neurologists aims to streamline differential diagnosis

When and how a multidisciplinary palliative care clinic can fill unmet needs for this population
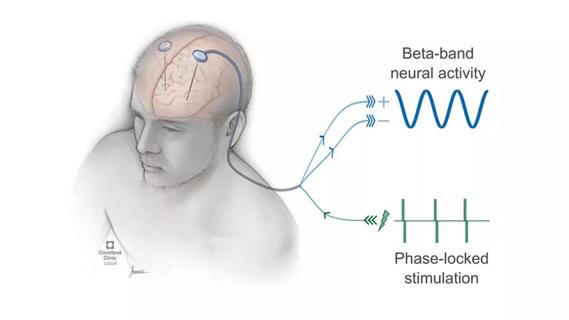
Research project will leverage insights into neural circuits to advance DBS technology