Systems genetics approach sets stage for lab testing of simvastatin and other candidate drugs
Researchers from the Cleveland Clinic Genome Center have successfully applied advanced artificial intelligence (AI) genetics models to Parkinson’s disease (PD). Their research, published in npj Parkinson’s Disease (2025 Jan 22;11:22), identified potential risk genes as well as several FDA-approved drugs that could potentially be repurposed for treatment of PD.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The investigators used a systems biology approach employing AI to integrate and analyze multiple forms of information from genetic, proteomic, pharmaceutical and patient datasets to identify patterns that may not be obvious from analyzing one form of data on its own.
Study lead and Genome Center Director Feixiong Cheng, PhD, is a leading systems biology researcher and has previously developed multiple AI frameworks to identify potential new or repurposed therapies for Alzheimer’s disease.
“Parkinson’s disease is the second most common neurodegenerative disorder, right after dementia, but we don’t have a way to stop or slow its progression in the millions of affected people worldwide,” says study first author Lijun Dou, PhD, a postdoctoral fellow in Dr. Cheng’s Genomic Medicine lab. “The best we can currently accomplish is managing symptoms as they appear. There is an urgent need to develop or identify new disease-modifying therapies for Parkinson’s disease.”
Developing compounds that halt or reverse the progression of PD is especially challenging because the field is still identifying which genes cause which PD symptoms when mutated, Dr. Dou explains.
“Many of the known genetic mutations associated with Parkinson’s disease are in noncoding regions of our DNA, not in actual genes,” she says. “We know that variants in noncoding regions can, in turn, impact the function of different genes, but we don’t know which genes are impacted in Parkinson’s disease.”
Using their integrative AI model, the research team was able to cross-reference genetic variants associated with PD with multiple brain-specific DNA and gene expression databases. This allowed the team to infer which, if any, specific genes in the brain are affected by variants in noncoding regions of DNA. The researchers then combined the findings with protein and interactome datasets to determine which of the genes they identified affect other proteins in the brain when mutated. They found several potential risk genes (such as SNCA and LRRK2), many of which are known to cause inflammation in the brain when dysregulated.
Advertisement
The research team next explored whether any drugs on the market could be repurposed to target the identified genes.
“It takes an average of 15 years of rigorous safety testing for a newly discovered or developed compound to be approved for clinical use as a medication,” Dr. Cheng explains. “Individuals currently living with Parkinson’s disease can’t afford to wait that long as their condition continues to progress. If we can use drugs that are already FDA-approved and repurpose them for Parkinson’s disease, we can significantly reduce the time needed to give patients more options.”
By integrating their genetic findings with available pharmaceutical databases, the team found multiple candidate drugs. They then referenced electronic health records to see if there were any differences in PD diagnoses among individuals taking the identified drugs relative to the general population. For example, individuals who had been prescribed the cholesterol-lowering medication simvastatin were significantly less likely to receive a PD diagnosis in their lifetime.
Dr. Cheng says the next step is to test the potential of simvastatin — along with several immunosuppressive and anti-anxiety medications also identified as warranting further study — for treating PD in lab models.
“Using traditional methods, any of the steps we took to identify genes, proteins and drugs would be very resource- and time-intensive tasks,” Dr. Dou says. “Our integrative network-based analyses allowed us to accelerate this process significantly and identify multiple candidates. This ups our chance of finding new solutions.”
Advertisement
This research was supported by grants from the National Institute on Aging (NIA) and National Institute of Neurological Disorders and Stroke (NINDS).
Advertisement
Advertisement

Various AR approaches affect symptom frequency and duration differently

Dopamine agonist performs in patients with early stage and advanced disease

Early assessment could affect clinical decision making

Study aims to inform an enhanced approach to exercise as medicine

New tool for general neurologists aims to streamline differential diagnosis

When and how a multidisciplinary palliative care clinic can fill unmet needs for this population
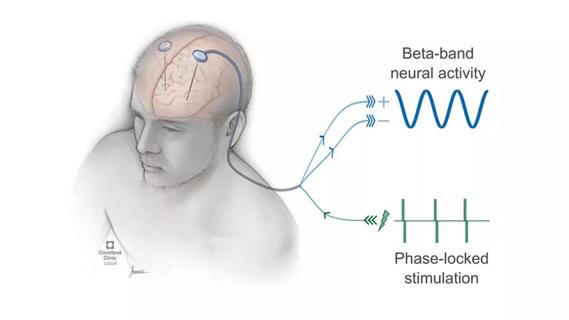
Research project will leverage insights into neural circuits to advance DBS technology

Randomized trial demonstrates noninferiority to therapist-led training