Large NIH-funded investigation is exploring this understudied phenomenon
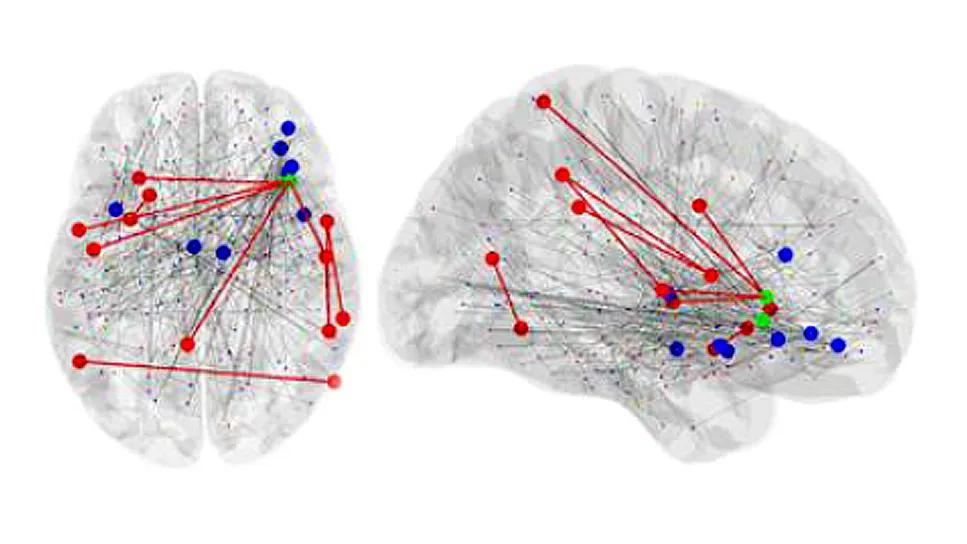
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/60e562d4-149c-42d5-b30e-53c64b528e9b/brain-connectivity-in-alzheimer-s-disease)
two brain images with colored dots and red line overlays
While Alzheimer’s disease is generally characterized as a steady progression of cognitive decline, some patients have marked periods of lucidity and normal function lasting for minutes or even days before lapsing back into their impaired state. These so-called cognitive fluctuations (CFs) may also manifest as periods of pronounced confusion or increased somnolence. Strikingly, CFs are even more common in dementia with Lewy bodies (DLB) and are even a core feature in its clinical diagnosis.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Cleveland Clinic neurologist Jagan Pillai, MD, PhD, Director of the Cleveland Alzheimer’s Disease Research Center, was recently awarded a $4.9 million research grant from the National Institutes of Health (NIH) to lead a multicenter research project over four years focused on characterizing CFs in Alzheimer’s disease and DLB.
“CF is an underrecognized yet clinically significant symptom that lacks objective measures,” Dr. Pillai explains. “A better understanding of these spontaneous changes in cognition, attention, arousal and function could potentially improve diagnosis, monitoring and clinical trials for both Alzheimer’s disease and DLB.”
Up to now, costly drug development and clinical trials for Alzheimer’s disease have led to only modest clinical gains. Dr. Pillai points out that a difficulty in studying this disease is the intra- and interindividual variability in clinical presentations and rates of cognitive decline. Variability between patients, he explains, may reflect underlying differences in the disease, potentially confounding clinical trial results by mixing distinct populations. At the individual level, CFs could cause inconsistent observations and answers to assessments taken periodically, also skewing results.
CFs are poorly characterized, with no identified biomarkers or tools to measure their occurrence. Relying mostly on observer report, they are found in 30% to 60% of people with early DLB. They are less common in Alzheimer’s disease, and individuals who experience them are likelier to be diagnosed with mild rather than more severe disease.
Advertisement
“CFs offer a window into the brain — when patients become lucid, it tells you that some normal functioning is preserved,” Dr. Pillai says. “If we understood them better, perhaps we could modulate something, maybe with exercise or medications, to help periods of clarity last longer or occur more consistently.”
His newly funded research is using a variety of measures to try to objectively and reliably characterize CFs to better understand how they occur.
The study is a Cleveland Clinic-led collaboration with VA Puget Sound in Seattle and University of California San Diego. The sites are currently recruiting a total of 108 participants, aged 50 to 90 years, evenly divided between patients with biomarker-confirmed Alzheimer’s disease and biomarker-confirmed DLB. All sites are conducting cognitive and physiological testing. In addition, University of Washington is contributing advanced data analytics.
Participants are being tracked for up to four years and will undergo annual informant-based questionnaires and neurocognitive measurements of CF. These will be compared with a variety of real-time tools, including:
“We anticipate that the real-time tools will help identify objective biomarkers reflecting the presence, frequency and stability of CF,” Dr. Pillai says. “We expect them to be more reliable than informant-based questionnaires, which are currently used to capture the presence of CFs.”
Advertisement
Dr. Pillai highlights several ways this research can contribute to the understanding of Alzheimer’s disease:
Dr. Pillai notes that this research involves the most comprehensive investigation to date focused on CFs, using the largest cohort of patients over the longest period of time.
Advertisement
“This novel tack in studying cognitive fluctuation in DLB and Alzheimer’s disease shows great promise by providing real-time physiological information for a poorly understood but potentially important phenomenon,” Dr. Pillai says. “I expect it will help move the field forward in innovative and significant new directions.”
Image at top: Brain images showing differences in connectivity between CF-positive and CF-negative groups.
Advertisement
Advertisement
An expert talks through the benefits, limits and unresolved questions of an evolving technology
How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits
Observational evidence of neuroprotection with GLP-1 receptor agonists and SGLT-2 inhibitors
Genomic study lays groundwork for insights into potential biomarkers and therapeutic strategies
Proteins related to altered immune response are potential biomarkers of the rare AD variant
Alzheimer’s studies delve into sex-related variances in the expression of the disease
Validated scale provides a method for understanding how lifestyle may protect against Alzheimer's
Collaborative approach may reduce distress caused by neuropsychiatric symptoms