Pathogenesis and optimal management of acute kidney injury in COVID-19
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b73c3fa8-55e1-4fdb-954f-771f26b0aca0/650x450-Covid-and-Kidneys_jpg)
650×450-Covid-and-Kidneys
By Mohamed Hassanein, MD, Yeshwanter Radhakrishnan, MD, John Sedor, MD, Tushar Vachharajani, MD, Vidula T. Vachharajani, MD, Joshua Augustine, MD, Sevag Demirjian, MD, and George Thomas, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
COVID-19 is primarily considered a respiratory illness, but the kidney may be one of the targets of SARS-CoV-2 infection, since the virus enters cells through the angiotensin-converting enzyme 2 (ACE2) receptor, which is found in abundance in the kidney. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), gains entry into target cells through the ACE2 receptors. ACE2 receptors are present in the kidneys as well as the lungs, heart, and intestinal cells.1-8 This article discusses the pathogenesis and optimal management of acute kidney injury (AKI) in COVID-19.
The renin-angiotensin system plays an important role in human physiology. Angiotensin I is cleaved from angiotensinogen by renin and converted to angiotensin II by ACE. Angiotensin II causes systemic vasoconstriction and also enhances inflammation, endothelial cell dysfunction, oxidative stress, collagen synthesis in fibroblasts, and fibrosis in target organs.7-9 ACE2 is a counter-regulatory enzyme that breaks down angiotensin II to form angiotensin 1–7, which mediates vasodilation and attenuates angiotensin II-mediated inflammation.7
SARS-CoV, the virus that caused the SARS epidemic in 2003, downregulates expression of ACE2 after it enters the cell, and without the counter-regulatory effects of ACE2, the deleterious effects of angiotensin II are believed to lead to lung disease, including severe acute respiratory distress syndrome (ARDS).7-10
The kidney has an abundance of ACE2 receptors and therefore may be one of the primary targets of SARS-CoV-2 infection.7 ACE2 is expressed in the kidney much more than in the lungs, specifically on the brush border apical membrane of the proximal tubule and also at lower levels in the podocytes.11 Virus particles were observed in the tubular epithelium and in podocytes in an autopsy series of COVID-19 patients.12 TMPRSS2 is robustly expressed in the distal nephron but not the proximal tubule; it is unclear if other transmembrane serine proteases in the proximal tubule can mediate the priming step.8
Advertisement
AKI in COVID-19 is also associated with a higher risk of death.13-21 A systematic review and meta-analysis23 of six studies from China found that severe AKI in COVID-19 (defined as AKI stage 3 and AKI requiring kidney replacement therapy) was associated with a three-fold higher risk of death.
A U.S. study17 reported a mortality rate of 35% in patients with AKI; of those who died, 91% had stage 3 AKI. The mortality rate was 55% in those needing kidney replacement therapy. Another study19 showed an in-hospital mortality rate of 33.7% in those with COVID-19-associated AKI compared with 13.4% in those with AKI without COVID-19. Those with stage 3 AKI and COVID-19 had a 2.6-fold higher mortality rate than those with stage 3 AKI who did not have COVID-19.
There are limited data on the long-term prognosis of COVID-19 patients with AKI. A single-center study from China reported that although the mortality rate was high in patients with AKI, nearly half of the patients recovered from AKI within three weeks of onset of infection.21 A U.S. study17 reported a median creatinine level of 1.70 mg/dL (interquartile range 0.96, 3.50) at the time of discharge in patients with AKI, and 91% of hospitalized patients who needed kidney replacement therapy were still on it at the time of study censoring. Another study from the U.S. showed that fewer COVID-19 AKI patients recovered renal function than AKI patients without COVID-19 (42.3% vs 68.5%).19
Proteinuria and hematuria have been reported in patients with COVID-19, but their significance and impact on mortality are not yet known. An early report from China13 found proteinuria in 43.9% and hematuria in 26.7% of patients. Subsequent studies from the U.S. reported 2+ or 3+ blood in 46% of patients, and 2+ or 3+ protein in 42% of patients on urine dipstick analysis. Another study showed proteinuria in 87% of patients.17-20
Advertisement
While the effects of ACE2 expression and viral entry on proteinuria are unknown, it is speculated that proteinuria could be related to viral replication, particularly in the podocytes.11
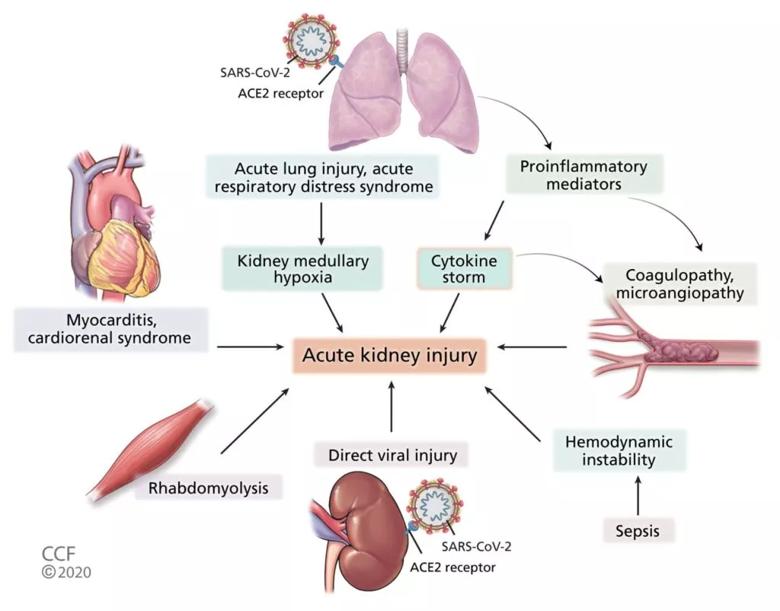
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e621faa5-ef13-4ab7-82c9-88ae46678d40/F1_large-1-1024x802_jpg)
Pathophysiology of acute kidney injury in COVID-19 (ACE2 = angiotensin-converting enzyme 2; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2).
| Cause | Supporting evidence |
|---|---|
| Prerenal (volume depletion) | Increased blood urea nitrogen: creatinine ratio (> 20), urine sodium < 20 mmol/L, fractional excretion of sodium < 1%Urine sediment may show hyaline casts |
| Acute tubular injury | Urine sodium > 20 mmol/L, fractional excretion of sodium > 1%Urine sediment with granular or muddy brown casts |
| Acute interstitial nephritis | Rash, eosinophilia, white blood cells on urine microscopyUrine sediment with white blood cell casts (urine eosinophils are not sensitive or specific) |
| Postrenal (obstruction) | Bladder scan with high postvoid residual volume, oliguria improving with Foley catheter placementKidney ultrasonography showing hydronephrosis |
| Rhabdomyolysis | Increased serum creatine kinase and myoglobin in urinePositive urine dipstick for blood, no red blood cells on microscopy |
| Abdominal compartment syndrome | Increased intra-abdominal pressure (> 20 mm Hg) |
| Coagulopathy | Elevated prothrombin time, partial thromboplastin time, D-dimer, fibrinogen |
| Cardiorenal syndrome | Jugular venous distention, low ejection fraction on echocardiography, urine sodium < 20 mmol/L |
| Cause | |
| Prerenal (volume depletion) | |
| Supporting evidence | |
| Increased blood urea nitrogen: creatinine ratio (> 20), urine sodium < 20 mmol/L, fractional excretion of sodium < 1%Urine sediment may show hyaline casts | |
| Acute tubular injury | |
| Supporting evidence | |
| Urine sodium > 20 mmol/L, fractional excretion of sodium > 1%Urine sediment with granular or muddy brown casts | |
| Acute interstitial nephritis | |
| Supporting evidence | |
| Rash, eosinophilia, white blood cells on urine microscopyUrine sediment with white blood cell casts (urine eosinophils are not sensitive or specific) | |
| Postrenal (obstruction) | |
| Supporting evidence | |
| Bladder scan with high postvoid residual volume, oliguria improving with Foley catheter placementKidney ultrasonography showing hydronephrosis | |
| Rhabdomyolysis | |
| Supporting evidence | |
| Increased serum creatine kinase and myoglobin in urinePositive urine dipstick for blood, no red blood cells on microscopy | |
| Abdominal compartment syndrome | |
| Supporting evidence | |
| Increased intra-abdominal pressure (> 20 mm Hg) | |
| Coagulopathy | |
| Supporting evidence | |
| Elevated prothrombin time, partial thromboplastin time, D-dimer, fibrinogen | |
| Cardiorenal syndrome | |
| Supporting evidence | |
| Jugular venous distention, low ejection fraction on echocardiography, urine sodium < 20 mmol/L |
| Drug | Mechanism of action | Evidence, comments | Possible nephrotoxicity |
|---|---|---|---|
| Antiviral therapy | |||
| Chloroquine, hydroxychloroquine | Prevent glycosylation of host receptors and inhibit viral entry into host cellsImmunomodulatory effect through inhibiting cytokine production | Initially thought to improve viral clearance and disease duration60,61 but evidence is increasingly unsupportiveThe emergency use authorization of hydroxychloroquine for severe COVID-19 was revoked in June 2020, as potential risks outweighed the benefits62 | Podocytopathy of the kidney mimicking Fabry disease (rare)57 |
| Lopinavirritonavir | Inhibits 3-chymotripsin-like protease | Antiretroviral combination drug approved for treatment of human immunodefi ciency virus infectionNo difference in viral clearance, mortality63No benefit for patients with severe COVID-19 compared with standard care63 | Reversible acute kidney injury57 |
| Ribavirin and favipravir | Inhibit RNA polymerase and inhibit viral replication | Favipravir is currently being evaluated in clinical trials in the United StatesNo prospective data to support use of ribavirin | |
| Remdesivir | Inhibits RNA polymerase and inhibits viral replication | Possible improvement in oxygen support status in severe COVID-19 with remdesivir58Use of remdesivir in COVID-19 patients was associated with shortened time to recovery, but overall 14-day mortality rate was not significantly different compared with placebo66Emergency use authorization issued for use in severe COVID-19 and recently expanded use to include all hospitalized patients with COVID-19 regardless of severity.56,57 | Potential mitochondrial toxicity with remdesivir57 |
| Immunomodulatory and anti-inflammatory therapy | |||
| Corticosteroids | Decrease Inflammation and decrease lung injury | Unpublished analysis from the United Kingdom showed a reduction in 28-day mortality rate in patients with severe COVID-19 on mechanical ventilation with the use of dexamethasone65 | |
| Tocilizumab, sarilumab | Monoclonal antibodies against interleukin 6 receptor; decrease cytokine storm | Repeated doses of tocilizumab may be required to decrease interleukin 6 levels7,59Tocilizumab is recommended by the Infectious Diseases Society of America only in the context of a clinical trial72 | |
| Convalescent plasma, intravenous immunoglobulin | Viral antibodies from previously infected and recovered patients | Clinical improvement in 5 critically ill patients with COVID-1968High-dose intravenous immunoglobulin reportedly effective in case series with severe COVID-1969Convalescent plasma has been granted emergency use authorization for hospitalized patients with COVID-19.58 | Proximal tubular injury with intravenous immunoglobu |
| Drug | |||
| Antiviral therapy | |||
| Chloroquine, hydroxychloroquine | |||
| Mechanism of action | |||
| Prevent glycosylation of host receptors and inhibit viral entry into host cellsImmunomodulatory effect through inhibiting cytokine production | |||
| Evidence, comments | |||
| Initially thought to improve viral clearance and disease duration60,61 but evidence is increasingly unsupportiveThe emergency use authorization of hydroxychloroquine for severe COVID-19 was revoked in June 2020, as potential risks outweighed the benefits62 | |||
| Possible nephrotoxicity | |||
| Podocytopathy of the kidney mimicking Fabry disease (rare)57 | |||
| Lopinavirritonavir | |||
| Mechanism of action | |||
| Inhibits 3-chymotripsin-like protease | |||
| Evidence, comments | |||
| Antiretroviral combination drug approved for treatment of human immunodefi ciency virus infectionNo difference in viral clearance, mortality63No benefit for patients with severe COVID-19 compared with standard care63 | |||
| Possible nephrotoxicity | |||
| Reversible acute kidney injury57 | |||
| Ribavirin and favipravir | |||
| Mechanism of action | |||
| Inhibit RNA polymerase and inhibit viral replication | |||
| Evidence, comments | |||
| Favipravir is currently being evaluated in clinical trials in the United StatesNo prospective data to support use of ribavirin | |||
| Possible nephrotoxicity | |||
| Remdesivir | |||
| Mechanism of action | |||
| Inhibits RNA polymerase and inhibits viral replication | |||
| Evidence, comments | |||
| Possible improvement in oxygen support status in severe COVID-19 with remdesivir58Use of remdesivir in COVID-19 patients was associated with shortened time to recovery, but overall 14-day mortality rate was not significantly different compared with placebo66Emergency use authorization issued for use in severe COVID-19 and recently expanded use to include all hospitalized patients with COVID-19 regardless of severity.56,57 | |||
| Possible nephrotoxicity | |||
| Potential mitochondrial toxicity with remdesivir57 | |||
| Immunomodulatory and anti-inflammatory therapy | |||
| Corticosteroids | |||
| Mechanism of action | |||
| Decrease Inflammation and decrease lung injury | |||
| Evidence, comments | |||
| Unpublished analysis from the United Kingdom showed a reduction in 28-day mortality rate in patients with severe COVID-19 on mechanical ventilation with the use of dexamethasone65 | |||
| Possible nephrotoxicity | |||
| Tocilizumab, sarilumab | |||
| Mechanism of action | |||
| Monoclonal antibodies against interleukin 6 receptor; decrease cytokine storm | |||
| Evidence, comments | |||
| Repeated doses of tocilizumab may be required to decrease interleukin 6 levels7,59Tocilizumab is recommended by the Infectious Diseases Society of America only in the context of a clinical trial72 | |||
| Possible nephrotoxicity | |||
| Convalescent plasma, intravenous immunoglobulin | |||
| Mechanism of action | |||
| Viral antibodies from previously infected and recovered patients | |||
| Evidence, comments | |||
| Clinical improvement in 5 critically ill patients with COVID-1968High-dose intravenous immunoglobulin reportedly effective in case series with severe COVID-1969Convalescent plasma has been granted emergency use authorization for hospitalized patients with COVID-19.58 | |||
| Possible nephrotoxicity | |||
| Proximal tubular injury with intravenous immunoglobu |
*This information is subject to change as new findings are published.
Please note: This is an abridged version of an article originally published in the Cleveland Clinic Journal of Medicine. The article in its entirety, including a complete list of references can be found here.
Advertisement
Advertisement
Patients report improved sense of smell and taste
Clinicians who are accustomed to uncertainty can do well by patients
Unique skin changes can occur after infection or vaccine
Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition
As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients
Early results suggest positive outcomes from COVID-19 PrEP treatment
Could the virus have caused the condition or triggered previously undiagnosed disease?
Five categories of cutaneous abnormalities are associated with COVID-19