Anti-IL-6 and anti-IL-1 agents: Center stage for COVID-19 stage 3 disease

Knowledge about the pathobiology of SARS-CoV-2 as it interacts with immune defenses is limited. SARS-CoV-2 is spread by droplets that come into contact with mucous membranes. COVID-19 is characterized by 2 or 3 stages: most patients who recover experience 2 stages of illness commencing with an asymptomatic or paucisymptomatic incubation period, followed by a nonsevere symptomatic illness lasting for several weeks, occurring in about 80% of those infected. In the remainder, a third phase marked by a severe respiratory illness, often accompanied by multisystem dysfunction, coagulopathy, and shock is observed. This phase of the illness is characterized by hypercytokinemic inflammation and is often referred to as “cytokine storm.” While the immunopathogenesis remains unclear, prospects of treating this severe phase of the illness with immunotherapy are evolving, with some treatments showing promise.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
As we learn about COVID-19, we recognize that there are gaping holes in our knowledge of the pathobiology of SARS-CoV-2 as it interacts with our immune defenses. Epidemiologically, we know that most people, especially young and healthy ones, do quite well at defending themselves from this infection and that even those with severe disease tend to recover without sequelae. We also know that not everyone has a relatively benign disease course and that risk factors for progression are dominated by age and comorbidities, especially cardiovascular dis ease, diabetes, and obesity.
While some of these clinical findings seem to have face-validity, others are not so clear. Why is age such a dominant risk factor, but on the other hand, why do some young, otherwise seemingly healthy individuals succumb to the infection? We do not yet have complete answers to these questions.
To tackle this problem, we must first examine what is known about the interaction between the pathogen and the host immune system. SARS-CoV-2 is spread by droplets that come into contact with mucous membranes. Interestingly, not all individuals who are exposed acquire the infection. Once a person is infected, the disease progresses through 2 or 3 main stages (Figure 1).1-4
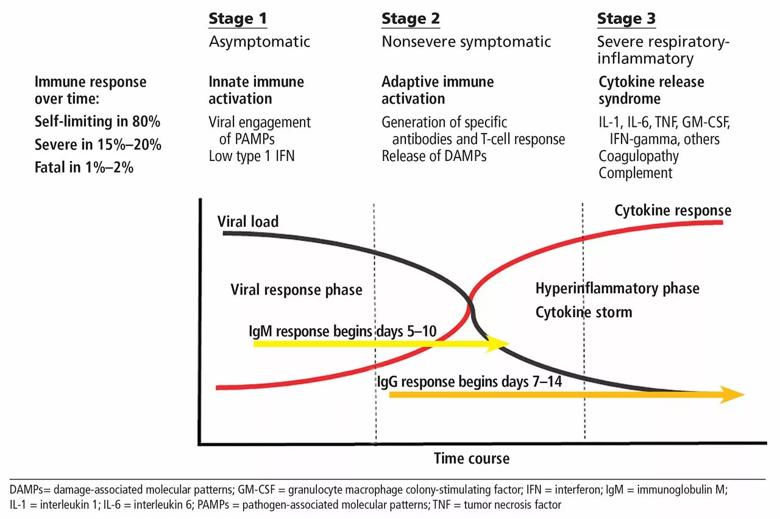
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e199fd8f-3e49-4ca6-99e1-8340004f2bc9/F1_large__jpg)
Why host antiviral defenses fail and why some patients go on to stage 3 is not yet clear, but attempts to reconcile these findings suggest that this progression may be driven by ongoing viral infection. In support of this hypothesis is a recent study documenting that SARS-CoV-2 infection actually induces a low interferon response, an immune pathway critical for antiviral defense, while at the same time inducing a strong inflammatory response, thus creating a perfect storm of continued viral replication and unbridled inflammation.5,6
Advertisement
The clinical state of patients with stage 3 disease is characterized by hypercytokinemic inflammation. This syndrome has variably been referred to as “cytokine storm.” The cytokine storm of COVID-19 bears similarities to other conditions that are also referred to under this umbrella, including primary hemophagocytic lymphohistiocytosis (HLH), as well as secondary forms such as macrophage activation syndrome (MAS) and secondary HLH, which are often encountered in the setting of autoimmunity, cancer, or viral infections.7,8 In COVID-19, and unlike in MAS or secondary HLH, the primary target organ is the lung, leading to an acute respiratory distress syndrome. While stage 3 COVID-19 is not secondary HLH or MAS, it does share features both clinically and pathologically.5
Recently, a variant of this cytokine storm has been described in children with COVID-19 and has been dubbed multisystem inflammatory syndrome in children (MIS-C).9
Laboratory features are quite similar among these disorders, with marked elevations of acute-phase reactants (eg, C-reactive protein, ferritin), lymphopenia, coagulation defects, and elevated levels of numerous inflammatory cytokines; prominent among them are interleukin 6 (IL-6), IL-1, IL-2, IL-7, IL-17, granulocyte macrophage-colony stimulating factor (GM-CSF), and tumor necrosis factor (TNF).10
Why there is an increased incidence of this inflammatory late-stage complication in select young individuals and more frequently in patients who are elderly and in those with comorbidities is poorly understood. Interestingly, though, in a study attempting to further understand why otherwise-healthy individuals can die from viral illness, 30% of patients dying from H1N1 influenza were found to carry single copies of genes commonly encountered in patients with HLH,11 suggesting a link between immune predisposition to HLH and outcome. We can also postulate that the chronic low-grade inflammation and an increase in self-reactivity that characterize the aging immune system also may contribute. Importantly, recent studies have shown that immunologic aging proceeds at different rates in different individuals; thus, mere chronologic age is, not surprisingly, a relatively crude predictor of COVID-19 progression.12
Advertisement
From a therapeutic perspective, there is a clear need for an effective antiviral agent that can prevent viral infection in exposed individuals and limit tissue damage in those with established disease (stages 1 and 2). In stage 3, in the absence of any effective antiviral therapy, we are relegated to supportive care. It is at this stage that the experimental use of agents designed to limit tissue damage driven by uncontrolled inflammation is being investigated. Given the similarities between stage 3 COVID-19 and other hypercytokinemic states, a variety of nonspecific immunosuppressive strategies have been proposed, such as glucocorticoids, hydroxychloroquine, colchicine, and other immunomodulators, as well as Janus kinase inhibitors and a number of targeted therapies directed at pivotal cytokines. For now, the experience with such agents largely consists of anecdotal case reports and small clinical trials.13
Among these therapies, agents that target IL-6 have perhaps generated the greatest enthusiasm. Numerous IL-6–targeting agents are being tested in COVID-19 including those targeting the IL-6 receptor (tocilizumab, sarilumab) and those targeting IL-6 itself (siltuximab, clazakizumab, and sirukumab). Two of these agents—tocilizumab (NCT04320615) and sarilumab (NCT04315298)—are already in advanced stages of multicenter randomized control trials, and data should be forthcoming soon.
Interest in IL-6 is strong, as it is a pleomorphic cytokine produced by both hematopoietic and viscerosomatic cells and has far-reaching effects on immune function and varied nonimmune physiologic processes.14 It is a key upstream driver of inflammation and has been successfully targeted therapeutically. IL-6 also has been shown to be a predictor of respiratory failure.
Advertisement
Of particular relevance for stage 3 COVID-19 disease, targeting IL-6 with tocilizumab is now indicated for treatment of cytokine storm accompanying chimeric antigen receptor (CAR)-T-cell therapy.15 Clinical support for advancing the study of IL-6 inhibition in COVID-19 has come from a variety of sources, including anecdotes from now-widespread off-label use of tocilizumab, case reports, and small series16 in which rapid reversal of laboratory and clinical parameters were reported. Balancing enthusiasm for such a strategy is the known pivotal role of IL-6 in host defense, particularly in defense against respiratory viruses.17
IL-1 is another inflammatory cytokine that could potentially be targeted to treat various cytokine storm syndromes. IL-1 is an upstream mediator of inflammation and is produced by the NLPR3 inflammasome; it has been incriminated in the pathogenesis of COVID-19, having been detected in lung tissue by a variety of techniques.18
As of this writing, 3 small nonrandomized case series have demonstrated benefit of IL-1 inhibition in COVID-19.18,19 The largest of these series,19 while suffering from the use of a retrospectively derived comparator group, demonstrated meaningful improvement in reducing the need for mechanical ventilation with the use of anakinra, a human IL-1 receptor antagonist. Anakinra has a short half-life, a large therapeutic window, and a well-established safety profile, and can be given by subcutaneous and intravenous routes.18 Large prospective trials are now under way and results are eagerly awaited.
Advertisement
Above all, there are serious considerations regarding untoward toxicity. Paramount among safety considerations is the potential for targeted therapies to suppress the host’s immune response and further limit failing antiviral defenses. Theoretically, the short-term use of such agents is likely to be less immunosuppressive than observed in long-term clinical use, but this hypothesis remains unproven. Concerns regarding the use of Janus kinase inhibitors is of particular note since they can further serve to suppress type I and III interferons, which are critical in antiviral defense.20Also critical is the potential risk associated with the timing of therapy. Administering treatment too early may compromise antiviral immunity, while waiting too long may risk irreversible organ damage.
Other novel therapies used alone or in combination include targeting GM-CSF, granulocyte-colony stimulating factor, and Janus kinase. These have been reviewed or mentioned in several excellent narrative reviews.5,8,13,21
Advertisement

Patients report improved sense of smell and taste

Clinicians who are accustomed to uncertainty can do well by patients

Unique skin changes can occur after infection or vaccine

Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition

As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients

Early results suggest positive outcomes from COVID-19 PrEP treatment

Could the virus have caused the condition or triggered previously undiagnosed disease?

Five categories of cutaneous abnormalities are associated with COVID-19