How a novel tissue marker makes a difference
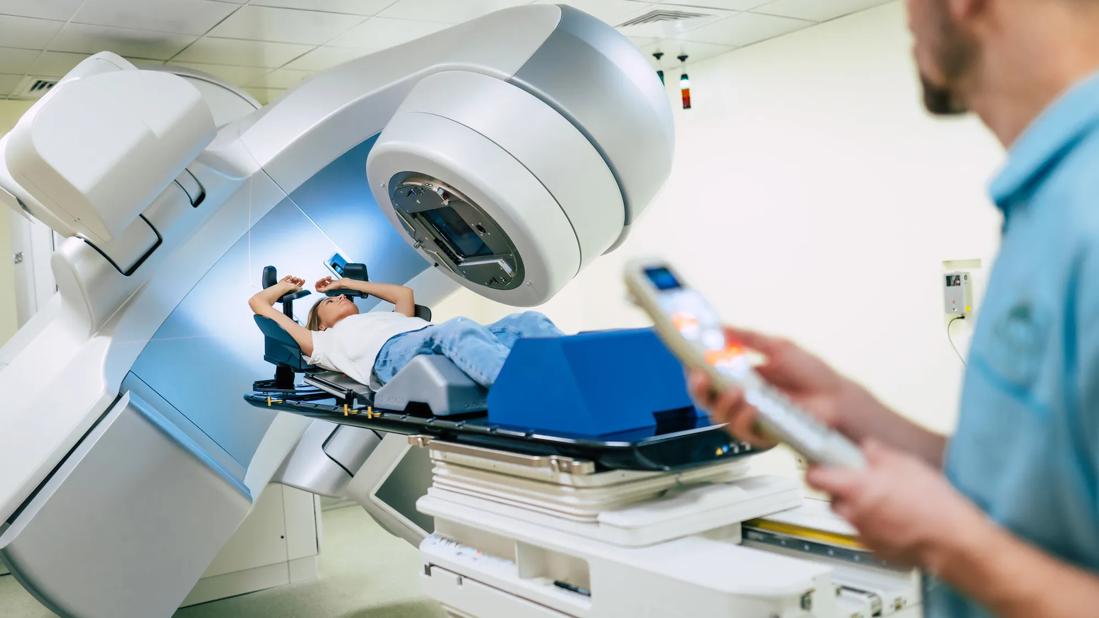
Breast surgeons and radiation oncologists at Cleveland Clinic are developing a new approach aimed at optimizing radiation therapy outcomes while reducing toxicity to normal breast tissue.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Partial breast irradiation (PBI) has emerged as an alternative to whole-breast irradiation for selected patients who have undergone breast-conserving surgery, as it has been shown to offer comparable local control with the potential for reduced toxicities.
Multiple techniques are available to deliver PBI. External beam radiation therapy (EBRT) is often a preferred approach as it eliminates the need for additional invasive procedures and has been shown to be cost-effective. However, there is concern that older EBRT PBI methods increase toxicity, including fibrosis of the breast. This is believed to be due to a combination of factors including the wide margins utilized as well as the use of 3D conformational radiation therapy as compared with newer planning techniques.
Now, Chirag Shah, MD, Director of Breast Radiation Oncology at Cleveland Clinic Cancer Center, and Zahraa Al-Hilli, MD, a breast surgeon in the Digestive Disease & Surgery Institute, are leading the effort to improve outcomes and minimize toxicity from partial breast irradiation (PBI) by incorporating a novel tissue marker, motion management and newer treatment planning techniques.
“You can use these new technologies together to improve the therapeutic ratio, that is, to keep recurrence rates low but also reduce side effects to the heart, lung and breast. As we continue to iterate this process and get better and better, the side effects go down and the treatment becomes shorter and shorter. When I started practicing 10 years ago, the duration of radiation was five to six weeks. Now we’re doing it in five days with fewer side effects and equal recurrence rates,” Dr. Shah says.
Advertisement
The newest addition to this treatment protocol is the incorporation of a novel tissue marker, the Veraform® (Videra Surgical, California). The product is a radiopaque “suture” that the breast surgeon places at the time of closure following lumpectomy, delineating the lumpectomy cavity that can be viewed with a radiation planning CT scan and/or mammogram.
According to Dr. Shah, “We can’t see typical sutures on radiation planning and treatment scans, but this marker allows me to see the edges of the surgical cavity and therefore serves as an accurate guide for radiation planning. That means we can treat a smaller area so that less normal tissue receives radiation.”
Dr. Al-Hilli, Dr. Shah and their team are currently conducting a study comparing normal tissue doses using the Veraform with those of previous cases without the marker in place. Results are expected in about a year.
At the time of radiation planning, patients are placed in an immobilization device and as well as a breathing coordinator system (Active Breathing Coordinator™, ABC, Elekta). With the ABC device, when a patient breathes in to about 70-80% of their maximal inspiration, known as deep inspiratory breath hold (DIBH), their chests lift up while the heart and lungs stay in place, increasing the distance from the radiation field.
“Breathing techniques are being used more and more as a way to protect the heart from all forms of breast radiation,” Dr. Shah says.
During treatment, the patient undergoes a 3D cone beam CT while the ABC device is used to reduce motion, allowing for alignment in three dimensions with the tissue marker. Three-dimensional imaging is being adopted increasingly in different types of cancer, including breast radiation.
Advertisement
Shah and colleagues have reduced expansions to 1.0-1.5 cm, similar to those used with non-EBRT PBI approaches, by using a combination of measures, including limiting breast motion with DIBH during simulation (radiation planning) and treatment while reducing setup error via cone beam CT during treatment, and allowing for alignment in three dimensions daily with the tissue marker. Finally, they are using intensity-modulated radiation therapy rather than 3D-conformal radiation therapy to increase the conformality of the dose delivered while reducing the amount of normal breast tissue treated. “It’s millimeter precision with these types of techniques,” Dr. Shah says.
Since the 2020 publication of Cleveland Clinic’s initial outcomes using image-guided PBI delivered with IMRT, more and more centers have been using a similar approach. Dr. Shah expects that the same will happen with use of the current protocol including the novel marker.
Cleveland Clinic, he says, “was one of the first in the region to do this type of radiation technique and has been a leader in terms of publishing on techniques, outcomes and cost-effectiveness, and in terms of presenting at national meetings while having a dedicated team that focuses on it. We continue to look at ways to reduce the heart and lung doses, improve patient comfort and improve long-term outcomes, including toxicity and cosmetic outcomes.”
Advertisement
Advertisement

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units