A study of patients with brain metastases treated with SRS
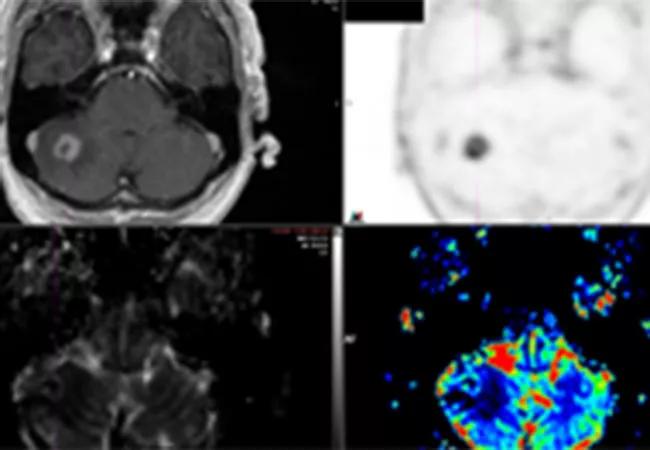
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dd842b7e-37f2-4a6d-9181-7d8acced7809/650x450-Chao_jpg)
650×450-Chao
Preliminary results from a recent Cleveland Clinic study demonstrate that 18F-fluciclovine PET/CT has the potential to distinguish radiation necrosis from tumor progression in brain tumors previously treated with stereotactic radiosurgery (SRS). The study was presented at the American Society of Radiation Oncology (ASTRO) 2020 Annual Meeting.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Oncologists often struggle to distinguish radiation-induced treatment effects from tumor recurrence in brain metastasis patients when looking at follow-up MRIs after radiosurgery.
Both conditions have similar morphological appearances on an MRI, and the patients can also exhibit similar symptoms: edema, memory loss, cognitive difficulties, seizures. The treatment for radiation necrosis (RN), however, is far less invasive than the treatment for tumor regrowth.
Several less invasive attempts to distinguish progression from RN are under investigation, including machine learning algorithms. “Our study examines an FDA approved radiotracer that is widely available for detecting prostate cancer recurrence,” says Samuel Chao, MD, radiation oncologist in the Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center of Cleveland Clinic Neurological Institute and Cleveland Clinic Cancer Center. “Approval and availability are two major hurdles that would already be surpassed if this method works.”
Patients with brain metastases frequently are treated by stereotactic radiosurgery, which offers excellent tumor control but can also cause RN.
Because it is difficult to distinguish between tumor recurrence and RN on an MRI, as many as 20 to 40 percent of all patients are subjected to multiple radiological studies, brain lesion biopsies and even resections for what is ultimately deemed RN. Distinguishing between the two is critical because the treatments for each are very different.
Brain biopsy is currently the only definitive test to determine whether an MRI image is RN or tumor recurrence, but such procedures comes with a cost. Brain biopsies are risky, often causing morbidity and mortality.
Advertisement
The study, designed with the help of Martin Tom, MD, a former Cleveland Clinic resident, included post SRS adult patients with brain metastases whose follow-up MRI brain was equivocal for tumor progression versus RN. A neuroradiologist grouped patients as equivocal but suspicious for RN, equivocal but suspicious for progression, or truly equivocal.
All patients underwent 18F-Fluciclovine PET/CT within 30 days of the equivocal MRI. Researchers collected PET data in list mode for 25 minutes after injection. Static image reconstruction occurred 10 to 25 minutes post injection, and a dynamic series of four five-minute frames were produced between five and 25 minutes post-injection. SUV max, SUV mean, SUV peak and normal brain SUV mean were documented for each lesion.
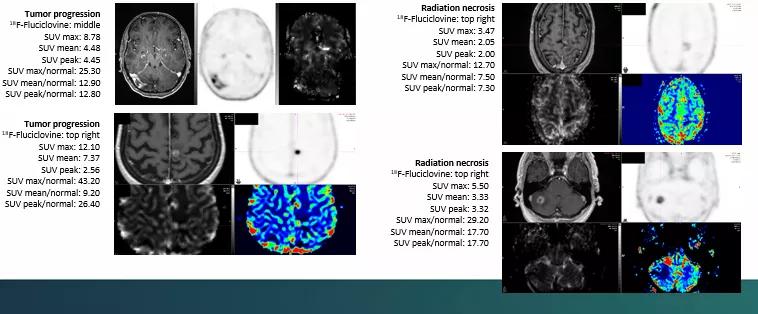
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fe96697e-e0d8-4cbc-b22d-8c938bf58b14/Chao-inset-2_png)
“The data needs further in-depth analysis, but it appears that SUV max may be helpful in distinguishing tumor recurrence versus radiation necrosis,” says Dr. Chao. “We are in the process of completing this study at our institution and other variables also may prove to be helpful in distinguishing between the two. Given the small number of patients in this study, the results will need to be further studied with a larger cohort of patients.”
In the patients studied, 18F-Fluciclovine PET/CT offered a wide range of metrics from which to derive data regarding lesions. It also had a low uptake in normal brain tissue, which points toward the ability to accurately discriminate tumor progression from RN.
“Right now, we are encouraged by these preliminary results,” says Dr. Chao. “A multisite, phase 3 trial is being developed, the results of which may help patients avoid invasive procedures in the future.”
Advertisement
Advertisement
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches