Patient-derived xenograft (PDX) models (immunodeficient mice engrafted with patients’ cancerous cells or tissues) have significantly enhanced cancer research in recent years. However, using PDX models to meet the urgent need for human cancer models to reliably predict clinical activity has proved challenging. Most cancer patients can’t wait months for the cells to become engrafted and grow and be used to test multiple drugs.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The average cancer grows too slowly to use PDX models to guide clinical decision-making. The patient will have received treatment long before the engraftment is ready for testing,” says Mohamed E. Abazeed, MD, PhD, a clinician and researcher in Cleveland Clinic’s Departments of Translational Hematology Oncology Research and Radiation Oncology.
At Cleveland Clinic, a 49-year-old female patient with metastatic clear cell adenocarcinoma of müllerian origin (an aggressive cancer that usually affects the cervix, endometrium and fallopian tubes and tends to disseminate rapidly), met the criteria for PDX-guided treatment: frequent upfront surgery providing ample donor tissue, rapid tumor proliferation and the absence of a definitive standard of care. A case study of her treatment appears in Precision Oncology.
At the time of diagnosis, the clear cell adenocarcinoma, which had originated in the small bowel, had metastasized to her liver and omentum. The median survival estimate is only several months for patients at a similar stage of the disease.
Several hours after her liver metastectomy, tissue was implanted into a mouse. Within 10 days, a tumor with the histopathologic features of clear cell adenocarcinoma developed. “By selecting cancers that grow aggressively, we can potentially develop PDX models in a timeframe that is clinically actionable,” says Dr. Abazeed, case study co-author with Roberto Vargas, MD, and Robert DeBernardo, MD, of the Gynecologic Oncology Division, Ob/Gyn & Women’s Health Institute. In the Cleveland Clinic inventory of 220 PDXs derived from multiple cancer types, 5.3 percent of successfully generated PDX have been harvested within two weeks of implantation, suggesting that the experimental design can be expanded to other rapidly proliferating cancers.
Advertisement
Genome-wide gene expression profiling showed high transcriptomic concordance of the matched donor tumor and the PDX. ERBB2 gene amplification was identified in the PDX and the levels of ERBB2 mRNA in the PDX and primary tumor were similar. These data indicated high genomic fidelity between the PDX and the donor tumor.
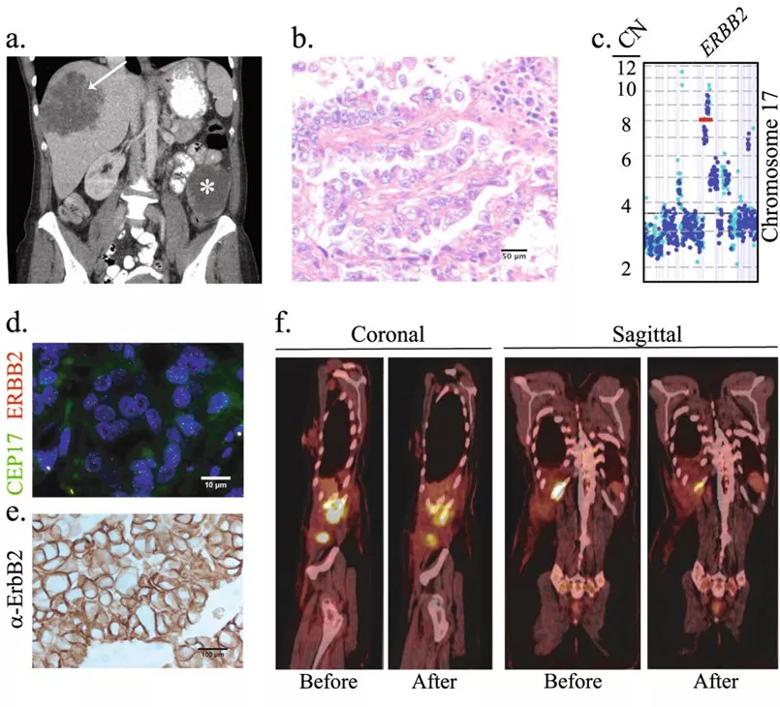
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4ca3f8ae-e9be-429f-a576-e0ac1301a7a6/abazeed-cs_805x730_jpg)
Clinical course and molecular profiling of the primary tumor. a Computed tomography scan obtained at the time of diagnosis. The 8.2 × 8.8 cm lobulated metastatic mass in the liver (arrow) and the 8.0 cm centrally necrotic primary tumor mass in the left mesentery (asterisk) are shown. b Representative image of an H&E stained section of the primary tumor. c Copy number count estimates from both exonic (blue) and intragenic or intronic (cyan) reads in Chromosome 17 are shown. d Representative FISH image of the primary tumor using ERBB2/CEP17 dual-color probes. The average ERBB2 signal copy number was 5.1 and the ERBB2/CEP17 ratio was 2.0. e Representative ErbB2 IHC image of the primary tumor. f Sagittal and coronal images of the PET/CT scans before and after treatment with three cycles of paclitaxel and neratinib (second-line treatment). The SUV maximum value for each lesion before and after treatment was, respectively: right lateral abdominal wall musculature, 11.7 and 6.6; posterior 11th rib, 11.2 and 4.1; and the soft tissue abutting the hepatic surgical site, 14.8 and 7.9. Originally published in Precision Oncology under license http://creativecommons.org/licenses/by/4.0/.]
Advertisement
Following the patient’s surgeries, CT scans revealed new right inguinal lymphadenopathy, an enlarging right chest wall mass (separate from the area of resection) and an abdominal incisional recurrence, indicating widespread metastatic disease.
The patient’s postoperative recovery allowed time to seek guidance from a multidisciplinary tumor board, who recommended the combination of nivolumab with either cisplatin, gemcitabine or an anti-ErbB2 agent. After initial engraftment, the PDX was implanted into 12 mice representing these four treatment cohorts. The PDX study indicated that the gemcitabine combination was the most effective at preventing tumor growth.
The patient ultimately received the combination of gemcitabine and nivolumab. In the third round of treatment in the PDX, progression was noted in one of the three mice. The treatment resistance was confirmed, and genetic testing showed that resistance was associated with gene expression changes that have been previously implicated in several classes of chemotherapeutics, including gemcitabine.
After five cycles of therapy (five months), the patient demonstrated a partial response in all known sites of disease and no evidence of new lesions. However, soon afterwards, as predicted by the PDX, the disease progressed to the perihepatic region, the right chest wall, the right lateral abdominal wall musculature and the brain.
Based on the results of another round of testing on the resistant PDXs, the patient received second-line treatment with concurrent paclitaxel with neratinib. She is alive with minimal residual disease 24 months past diagnosis, far longer than the average prognosis. “Using the PDX model, we were able to prospectively predict the patient’s response to first-line therapy and identify the most optimal second-line therapy,” says Dr. Abazeed.
Advertisement
An NIH-funded follow-up study is in the works. It will randomize patients to avatar-directed or standard- of-care therapy and compare outcomes. “We’re very excited about applying our avatar models to improve response rates in aggressive cancer types that have had very few advances in care. If we can even marginally improve the response rates for these generally recalcitrant tumors, that would represent a very significant advance for patients and their outcomes,” says Dr. Abazeed.
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors