Investigator speaks to potential applications beyond stroke
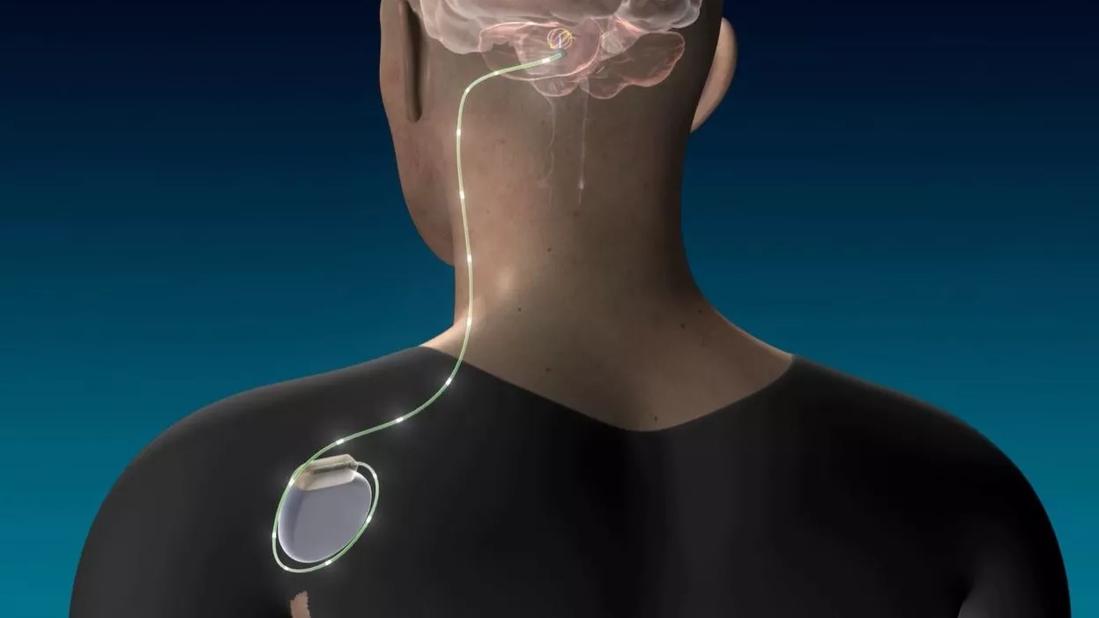
As previously reported on this blog, Cleveland Clinic researchers have launched the first clinical trial to examine the use of deep brain stimulation (DBS) to promote motor function recovery in disabled stroke survivors. The trial, launched in 2016 (a DBS device was implanted in the first patient in December), was awarded nearly $5 million in funding from the NIH’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The trial’s lead investigator, neurosurgeon Andre Machado, MD, PhD, recently shared some insights on the trial’s clinical implications in this video post. Dr. Machado explains the essentials of the trial, which patients are candidates, and how DBS for stroke recovery differs from neuromodulation for movement disorders.
“Our primary hypothesis is that by applying DBS to the connections between the cerebellum and cerebral cortex, we can facilitate the plasticity that occurs in the cortex around the stroke and thereby promote recovery of function beyond what physical therapy alone can do,” Dr. Machado notes. “We need more and better options to help the many patients who remain chronically disabled after a stroke.”
Now co-investigator Kenneth Baker, PhD, assistant staff in Cleveland Clinic’s Department of Neurosciences, speaks to some of the scientific implications of the study’s attempt to stimulate the brain’s dentatothalamocortical pathway to restore lost motor function. He also explains how Cleveland Clinic’s recent NIH BRAIN grant award is supporting the team’s work.
“We know that deep cerebellar stimulation promotes motor recovery in a preclinical model of cortical stroke,” says Dr. Baker. “Our goal is to advance this therapy to promote recovery of motor function in humans. This has the potential to be a significant advancement for the field.”
Check out his insights in the video below.
Video content: This video is available to watch online.
View video online (https://www.youtube.com/embed/EbJDJSQhxV8?feature=oembed)
Advertisement
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help