Advances toward biomarkers of progression are underway
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/58fa755c-8a01-4844-a028-abc673b39b23/17-CCC-4470-Rao-Prodromal-HD-650x450_jpg)
17-CCC-4470-Rao-Prodromal-HD-650×450
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Although formal diagnosis of Huntington’s disease (HD) is made at the appearance of unequivocal motor signs — usually between ages 35 and 50 — subtle motor, psychiatric and cognitive symptoms can occur decades prior to a manifest diagnosis. This period of time is referred to as prodromal HD (prHD).
With the development of treatments that stand to delay the onset or slow progression of HD symptoms, there is a strong need for outcomes that are sensitive to progressive neuronal dysfunction during the prodromal phase to evaluate the efficacy of therapies. Measures of functional (resting-state fMRI) and structural (diffusion MRI) connectivity are of great interest, as they may elucidate the effects of early striatal degeneration and other pathological processes on brain networks.
An interdisciplinary research team at Cleveland Clinic has recently pursued a series of investigations that demonstrate a largely disease burden-dependent functional reorganization of functional and structural brain networks in prodromal HD. Our team involves experts in neuropsychology, cognitive neuroscience, neurology, neuroradiology and MR physics and includes collaborations with experts at the University of California San Diego and University of Iowa. This post briefly recaps our findings and their potential to help pinpoint imaging biomarkers of prHD progression for use in therapeutic clinical trials.
For our studies, we have recruited HD at-risk participants (persons with a parent diagnosed with HD) who agreed to undergo genetic testing. Controls are individuals without an expansion in the HTT gene (gene-negative). Persons with an expansion in the HTT gene (gene-positive) who have not been diagnosed with manifest HD are further classified into low, medium or high prHD groups based on their probability of receiving a diagnosis of manifest HD within five years. This classification, which is a measure of disease burden, is based on a formula that accounts for the person’s age and the known inverse relationship between the length of cytosine-adenine-guanine (CAG) repeats in the HTT gene and age at diagnosis of manifest HD.
Advertisement
Our initial published work1 demonstrated that prHD is associated with abnormal interhemispheric interactions among motor areas and disturbances in the functional connectivity of the motor and visual centers. This study cross-correlated the temporal pattern of resting-state fMRI brain activity from a seed region, in this case the left primary motor cortex (M1), with every other region in the brain. In the cuneus and precentral gyrus, an increase in connectivity was observed in the low prHD group, suggesting compensatory changes in brain connectivity at the very earliest stage of the disease process. In contrast, connectivity from left M1 to the right cuneus, postcentral gyrus and precentral gyrus declined in the high prHD participants, a later disease stage event. These results suggest that reduced connectivity in these regions contributes to increased motor symptoms, visuomotor integration problems, and deficits in movement control as individuals approach a manifest diagnosis.
In a second study,2 we used an alternative analysis approach, referred to as the network-based statistic, to examine functional reorganization of whole-brain networks in prHD. Instead of selecting a seed region a priori as in our previous study, we examined patterns of change in functional connectivity by examining cross-correlations among 300 regions throughout the brain. We simultaneously demonstrated a pattern of weakened frontostriatal connections and strengthened frontal-posterior connections as a function of disease burden.
Advertisement
These results suggested a reconfiguration of networks, with increased frontal-parietal connections involving long-range pathways involved in attentional processes. These increased connections may enable the person with prHD to compensate cognitively for increasingly disrupted network connections between frontal and striatal areas. Importantly, this analysis provided a unique window into brain reorganization that was not related to brain atrophy or motor symptoms
In our most recent study,3 we used diffusion tensor imaging (DTI) to examine longitudinal changes in structural connectivity in prHD. DTI measures the integrity of white matter fiber tracts in the brain. We found that longitudinal changes in diffusivity, measured on an annual basis for two years, were localized to a white matter fiber tract called the superior fronto-occipital fasciculus (Figure). These changes were most prominent in individuals closer to a manifest diagnosis. Increases in motor symptoms across time were associated with greater changes in the superior fronto-occipital fasciculus diffusivity over time, in addition to atrophy of basal ganglia (putamen and caudate). These findings provided novel insights into longitudinal changes in different facets of structural brain connectivity in prHD.
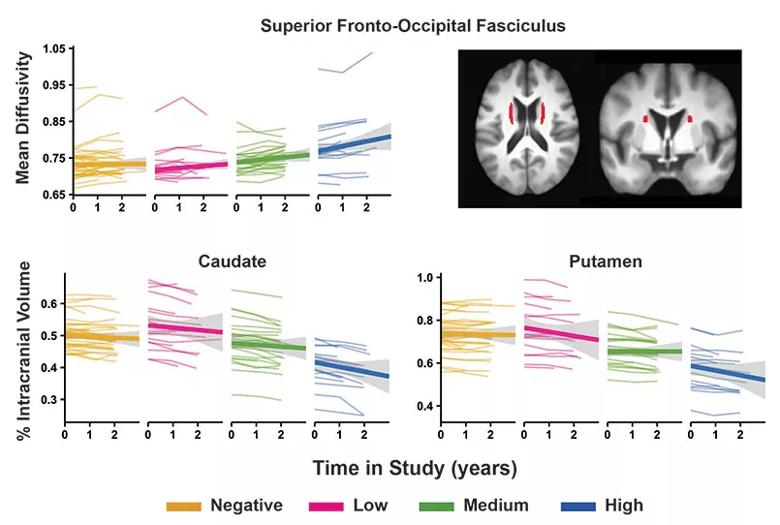
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7e8f5295-368f-4c20-8b0b-fde95f1187fa/17-CCC-4470-Rao-Prodromal-HD-inset_jpg)
Figure. Longitudinal trajectory of change in MRI variables in gene-negative and gene-positive individuals. Graphs display the individual trajectories of change over time for three MRI variables — mean diffusivity for the superior fronto-occipital fasciculus and volumes of the caudate and putamen — that showed a significant group-by-time interaction. The x-axis plots time in study, indexed in years. Graphs show the variability of individual trajectories (thin colored lines) around the group mean trajectory (wide colored lines) for the color-coded participant groups as shown in the key. Adapted, with permission, from Harrington et al,3 ©2016 International Parkinson and Movement Disorder Society.
Advertisement
Collectively, our results show for the first time a largely disease burden-dependent functional reorganization of functional and structural brain networks in prodromal HD. Both seed- and network-based analytic approaches provided a unique window into brain functional reorganization that was not related to brain atrophy or motor symptoms. Our longitudinal results have charted the course of functional changes to determine the most sensitive imaging biomarkers of disease progression for clinical trials aimed at preventing or slowing progression of the disease during the prodromal HD phase.
Dr. Rao holds the Ralph and Luci Schey Endowed Chair in Cleveland Clinic Lou Ruvo Center for Brain Health, Cleveland. This research was supported by grants from the National Institute of Neurological Disorders and Stroke (5U01NS082083) and the CHDI Foundation (Stephen M. Rao, principal investigator).
Advertisement
Advertisement
Guidance from the largest cohort of SEEG-confirmed insular epilepsy patients reported to date
Ethical guidance provides guardrails so medical advances benefit patients
OCEANIC-STROKE results represent long-sought advance in secondary stroke prevention
Two studies from Cleveland Clinic may help advance the technology toward broader clinical use
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
An argument for clarifying the nomenclature
An expert talks through the benefits, limits and unresolved questions of an evolving technology
Recommendations on identifying and managing neurodevelopmental and related challenges