A possible association between ACE2 and TMPRSS2 polymorphisms and COVID-19 susceptibility
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9d3952cd-a774-4f7e-ad14-74a016fbed36/coronavirus-GettyImages-1203801306-jpg)
20-CCC-1893680-SARS-CoV-2-Virus-Microvillie_650x450
“COVID-19 is strangely and tragically selective,” write the authors of a study recently published in BMC Medicine. “Human and genetic factors may contribute to the extremely high transmissibility of SARS-CoV-2 and to the relentlessly progressive disease observed in a small but significant proportion of infected individuals, but these factors are largely unknown.”
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The new Cleveland Clinic study has identified genetic factors that may influence susceptibility to COVID-19, which could guide personalized treatment.
While the majority of confirmed COVID-19 cases result in mild symptoms, the virus does pose a serious threat to certain individuals. Morbidity and mortality rates rise dramatically with age and co-existing health conditions, such as cancer and cardiovascular disease. However, even young and otherwise healthy individuals have unpredictably experienced severe illness and death. These clinical observations suggest that genetic factors may influence COVID-19 disease susceptibility, but these factors remain largely unknown.
In this study, a team of researchers led by Feixiong Cheng, PhD, Genomic Medicine Institute, investigated genetic susceptibility to COVID-19 by examining DNA polymorphisms (variations in DNA sequences) in the ACE2 and TMPRSS2 genes. ACE2 and TMPRSS2 produce enzymes (ACE2 and TMPRSS2, respectively) that enable the virus to enter and infect human cells.
Looking at ~81,000 human genomes from three genomic databases, they found 437 non-synonymous single-nucleotide variants in the protein-coding regions of ACE2 and TMPRSS2. They identified multiple potentially deleterious polymorphisms in both genes (63 in ACE2; 68 in TMPRSS2) that offer potential explanations for different genetic susceptibility to COVID-19 as well as for risk factors.
Several ACE2 variants were found to be associated with cardiovascular and pulmonary conditions by potentially altering the angiotensinogen-ACE2 interaction. In addition, germline deleterious variants in the coding region of TMPRSS2, a key gene in prostate cancer, were found to occur in different cancer types, suggesting that oncogenic roles of TMPRSS2 may be linked to poor outcomes with COVID-19.
Advertisement
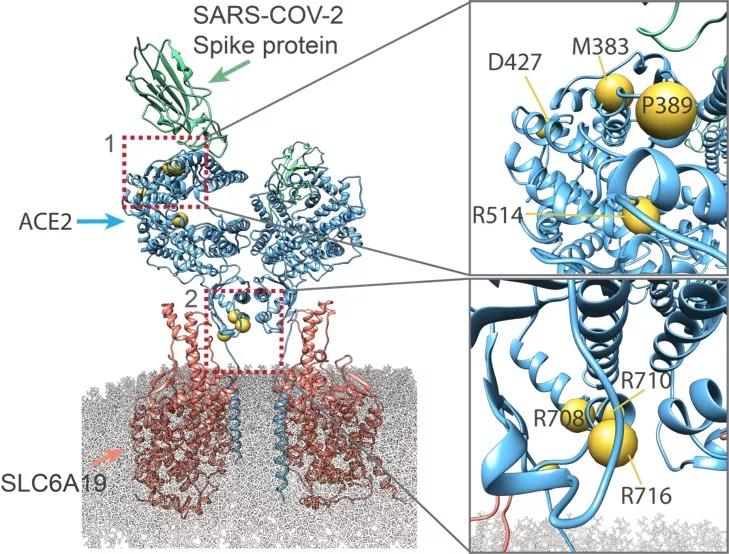
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b1118c5a-ba88-4f17-aa6a-6657b9d2caf7/news_1594823215_jpg)
Structural view of the coding-region variants in ACE2.
These findings demonstrate a possible association between ACE2 and TMPRSS2 polymorphisms and COVID-19 susceptibility, and indicate that a systematic investigation of the functional polymorphisms in ACE2 and TMPRSS2 among different populations could pave the way for precision medicine and personalized treatment strategies for COVID-19. However, all investigations in this study were performed in general populations, not with COVID-19 patient genetic data. Therefore, Dr. Cheng calls for a human genome initiative to validate his findings and to identify new clinically actionable variants to accelerate precision medicine for COVID-19.
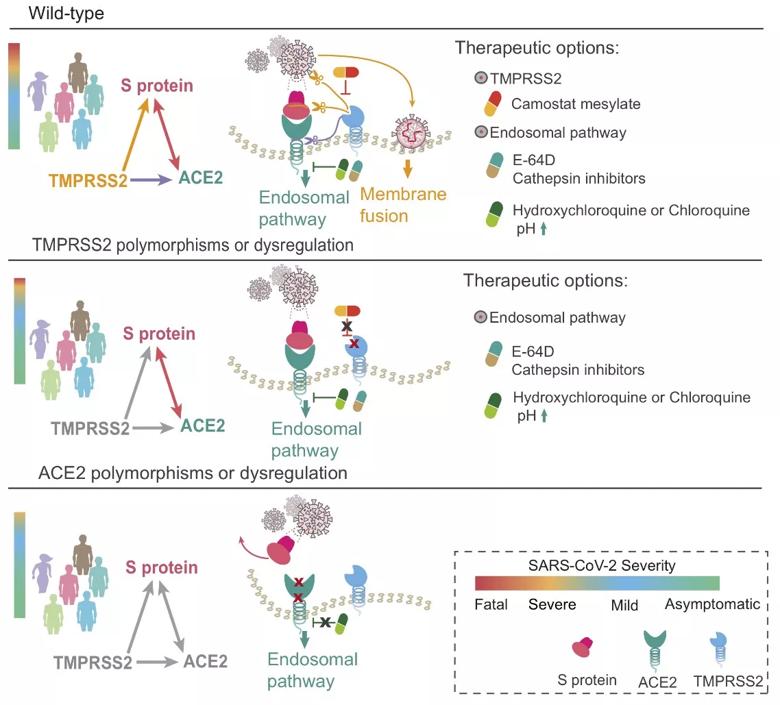
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d9623fed-071e-4ac5-9551-c551b68260a1/news_1594823218_jpg)
A proposed pharmacogenomics model of potential combination therapies (i.e., hydroxychloroquine, E-64D (a protease inhibitor), and camostat mesylate (an approved TMPRSS2 for treatment of chronic pancreatitis in Japan)) for COVID-19 by blocking ACE2 and TMPRSS2 across different populations with three genotypes.
“Because we currently have no approved drugs for COVID-19, repurposing already approved drugs could be an efficient and cost-effective approach to developing prevention and treatment strategies,” Dr. Cheng said. “The more we know about the genetic factors influencing COVID-19 susceptibility, the better we will be able to determine the clinical efficacy of potential treatments.”
This study was supported by the National Heart, Lung, and Blood Institute and the National Institute of Aging (both part of the National Institutes of Health) as well as the Cleveland Clinic VeloSano Pilot Program.
Advertisement
Advertisement
First full characterization of kidney microbiome unlocks potential to prevent kidney stones
Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.
Preclinical work promises large-scale data with minimal bias to inform development of clinical tests
Cleveland Clinic researchers pursue answers on basic science and clinical fronts
Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches
Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology