Cleveland Clinic physicians weigh in
Editor’s note: Article originally published in the Cleveland Clinic Journal of Medicine (2023; 90 [8]:475-481)
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Written by Ellen L. Ferraro, MD; Rebecca Chota Nelson, MD; and Paul C. Bryson, MD, MBA
The terms hoarseness and dysphonia are frequently used interchangeably. However, it is important to clarify that hoarseness is a patient-reported symptom, whereas dysphonia is the perception of altered voice quality noted by the clinician.1,2
Dysphonia results when there is a physiologic change to the properties of the vocal folds.2 This can be secondary to altered muscle tone (neurologic), impaired closure and mobility (paresis), increased bulk (e.g., tumor, polyp, cyst), mucosal vibratory changes (e.g., scar, inflammation, edema) or a combination.
In this article, we review basic laryngeal anatomy and function, causes of dysphonia, and current guidelines from the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS)1 on diagnosis and treatment of hoarseness and when to refer patients for specialist evaluation.
Hoarseness, either transient or prolonged, is a common complaint affecting as many as one-third of adults at least once in their lifetime.3 Unfortunately, only a fraction of affected individuals seek treatment because they believe it will resolve, they do not know treatment is an option or physicians do not inquire about perceptible vocal changes.3 While the majority of hoarseness is caused by benign pathology, up to 3% can be caused by malignant tumors and up to 8% by underlying neurologic disorders.2 Additionally, benign vocal-fold lesions such as cysts, polyps and nodules are treatable and are responsible for 10% to 30% of complaints.2 Thus, the cause of hoarseness cannot be determined without appropriate workup, and definitive treatment cannot be provided until a diagnosis is confirmed. Therefore, it is imperative that clinicians have guidance on how to manage, treat and refer these patients.
Advertisement
When patients are referred to otolaryngology specialists, flexible laryngoscopy may be performed in the office to directly visualize the larynx to aid in diagnosis and guide treatment (Figure 1). In-office laryngoscopy is a routine otolaryngologic procedure performed with the patient sitting upright and awake, without any sedation. A small, flexible camera is passed through the nasal cavity and advanced through the nasopharynx to view the laryngeal complex from above. It is well tolerated by adults and most children and allows clinicians a direct look at the base of the tongue, hypopharynx and larynx.
Videostroboscopy uses a similar camera but has a special light mechanism allowing for more detailed visualization of vocal-fold motion and function. However, it is not available in all otolaryngology offices.
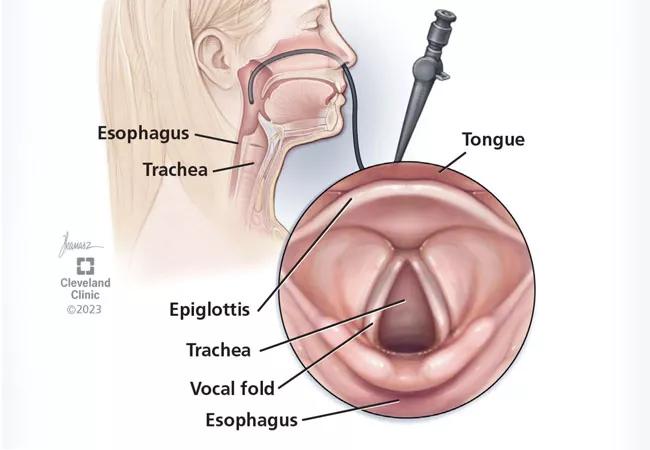
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/60679ae1-f915-4c1f-8caa-6e21f3d56a94/23-HNI-4138962-Hoarseness-ObserveOrRefer-CQD-hero_650x450_jpg)
Figure 1. Flexible laryngoscopy is a routine otolaryngologic procedure typically performed in the office for visualization of the larynx, to aid in diagnosis and guide treatment.
The laryngeal skeleton consists of three unpaired cartilages (thyroid, cricoid and epiglottic) and three paired cartilages (arytenoid, cuneiform, and corniculate).4 The thyroid cartilage is the largest and houses the vocal folds. From an anterior view, the vocal folds sit approximately midway between the inferior thyroid cartilage margin and the laryngeal prominence or “Adam’s apple” (Figure 2).
The muscles responsible for closing or adducting the vocal folds are the thyroarytenoid, lateral cricoarytenoid and interarytenoid, and the posterior cricoarytenoid is responsible for opening or abducting the folds. These muscles are all innervated by the recurrent laryngeal nerve.4 Mobility of the vocal folds resulting in hoarseness can therefore be affected by muscle or nerve dysfunction. While unilateral hypomobility and immobility may result in dysphonia, bilateral hypomobility and immobility can cause critical airway obstruction.
Advertisement
The “false” vocal folds (also called vestibular membrane or vestibular folds) sit superiorly to the “true” vocal folds (also called vocal cords) and are separated by the laryngeal ventricle (Figure 3). The true vocal folds generally protect the airway, modulate airflow and acoustics,5 and are highly sophisticated anatomic structures that are responsible for phonation. The true vocal folds consist of three specialized anatomic layers covering the thyroarytenoid muscle (also called the vocalis muscle): squamous epithelium, superficial lamina propria, intermediate lamina propria and deep lamina propria. Together, the intermediate and deep lamina propria make up the vocal ligament.6 The integrity of these layers is important because the vibration of the epithelial layer over the gelatinous layer of the superficial lamina propria produces smooth, clear phonation.6 Any disruption of these layers by tumor, fibrous nodules, cyst, scar or muscle atrophy may result in hoarseness.
The laryngeal skeleton is divided into three subsections based on the location of the true vocal folds. The area above the true vocal folds is referred to as the supraglottis, the area below the true vocal folds is the subglottis, and the horizontal plane of the true vocal folds is the glottis.6
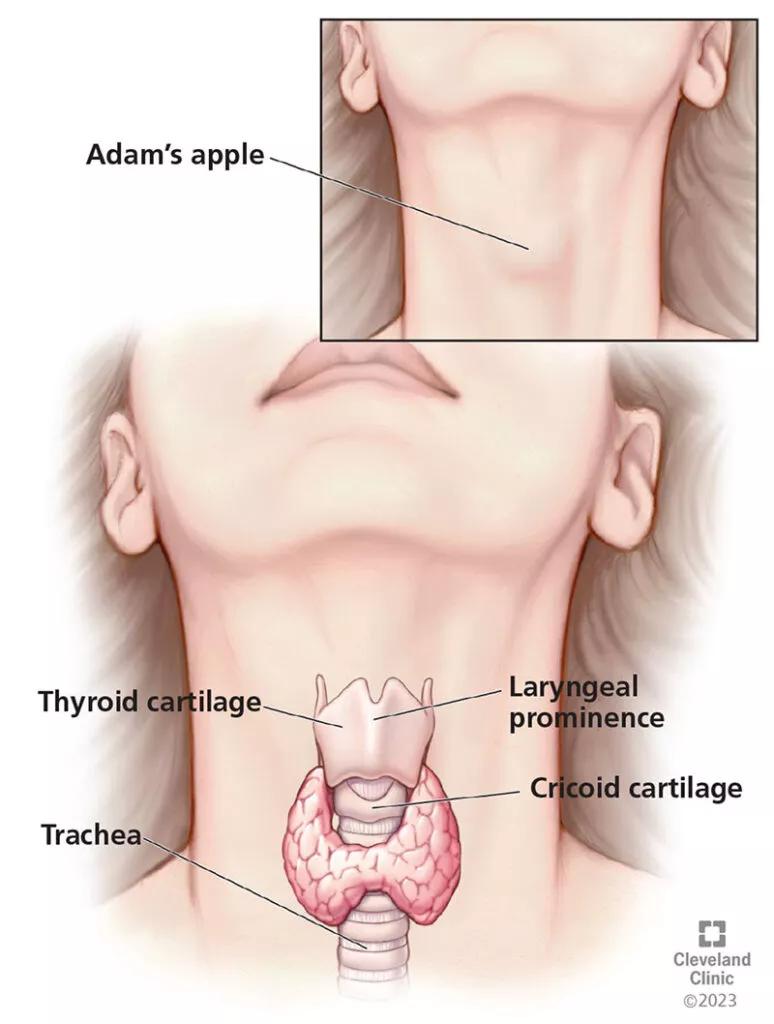
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/19ae28dd-ce85-49f0-914f-a1222127e80f/23-HNI-4138962-Hoarseness-ObserveOrRefer-CQD-inset1_650x450-774x1024_jpg)
Figure 2. The vocal folds sit approximately midway between the inferior thyroid cartilage margin and the laryngeal prominence or “Adam’s apple.”
The main functions of the larynx are respiration, airway protection and phonation.6 It takes great coordination for these mechanisms to synchronize so that patients may breathe, swallow, and phonate simultaneously. For respiration, the vocal folds open via action by the single laryngeal abductor muscle (posterior cricoarytenoid muscle).4 During expiration, the laryngeal adductor muscles work to pull the vocal folds partially toward the midline and modulate expiratory airflow.
Advertisement
The vocal folds protect the airway via multiple complex mechanisms and reflexes (such as coughing or the Valsalva maneuver).5 During phonation, the vocal folds posture so that they sit in the midline, allowing the folds to vibrate against each other and creating vocalization.5 The mucosal surface of the folds must line up straight and smooth to create a mucosal wave.6 Irregularities of the mucosa may cause vibration, leading to dysphonia.7 The frequency and pitch of the voice may be altered by contraction of the laryngeal muscles at different ratios, causing changes in length and position of the vibrating portion of the vocal folds themselves.5
A general understanding of the pathology that may cause dysphonia is helpful to clinicians for properly evaluating patients. The consequence of missing a diagnosis varies greatly depending on the severity and nature of the pathology. For the purpose of this review, we break down the major causes of dysphonia into functional, organic, neurologic and systemic.
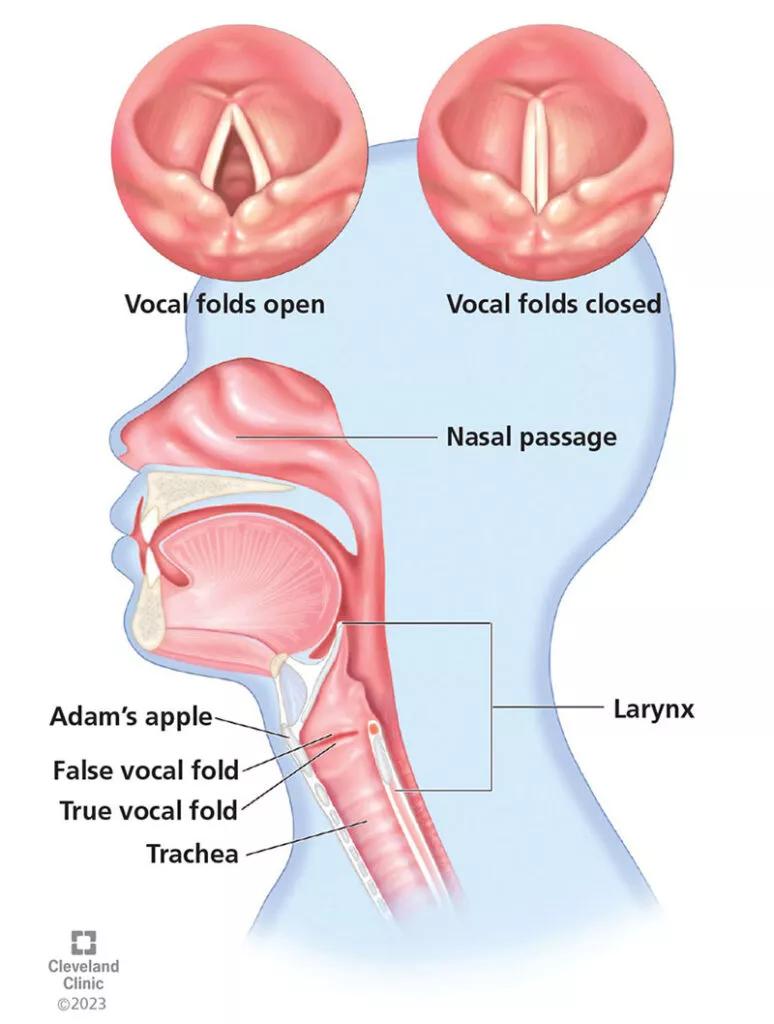
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/cd84d4b1-904a-4b50-a724-f634d45660c4/23-HNI-4138962-Hoarseness-ObserveOrRefer-CQD-inset2_650x450-774x1024_jpg)
Figure 3. The true vocal folds generally protect the airway and modulate airflow and acoustics, and their highly sophisticated anatomic structure impacts phonation. Although many people refer to the true vocal folds as vocal cords, they are anatomically not a cord.
Functional dysphonia is the alteration of vocal quality in the absence of anatomic or neurologic dysfunction. It is relatively common and present in 10% to 40% of patients referred to multidisciplinary voice clinics.8 It is predominantly seen in women and typically follows symptoms of upper respiratory infection. Stress, emotion, and psychologic conflict tend to exacerbate symptoms.8 Muscle tension dysphonia is the preferred term owing to presumed dysregulation of laryngeal muscle tension caused by either intrinsic or extrinsic laryngeal muscles. Laryngoscopic examination often reveals a “squeezing” or hyperfunction of the laryngeal complex. These patients are best treated with voice therapy with a voice-focused speech language pathologist.8
Advertisement
ACUTE AND CHRONIC LARYNGITIS
Acute laryngitis causes up to 40% of complaints of hoarseness, lasts up to two weeks, and most commonly is viral in origin.2 Alternatively, chronic laryngitis is long-standing and can have several causes, such as reflux, exposure to nicotine and noxious inhalants or inhaled corticosteroids, excessive alcohol or caffeine intake, and bacterial or fungal infection. The most common symptoms of chronic laryngitis overlap with other conditions and include dysphonia, pain, globus, cough, throat-clearing and dysphagia. Treatment includes avoidance of causative factors and treatment of underlying infectious or inflammatory conditions.2,9
BENIGN VOCAL-FOLD LESIONS
Vocal-fold nodules are bilateral fibrous swellings along the junction of the medial edge of the anterior and middle third of the true vocal folds.2 They act like calluses and prevent the propagation of a smooth mucosal wave, resulting in dysphonia. They are secondary to functional voice disorders and vocal abuse or misuse; 80% of patients will recover with voice therapy alone, while those with recalcitrant lesions may require surgical excision.2
Polyps and cysts also occur at the medial edge of the true vocal folds but are unilateral tissue proliferations that disrupt the mucosal wave and result in dysphonia. Unlike nodules, they typically do not resolve with therapy and do require surgical excision.2
Papillomas. Recurring respiratory papillomatosis is caused by human papillomavirus and leads to respiratory papillomas, which are exophytic lesions that grow along the laryngeal and sometimes tracheobronchial mucosa.2,10 When they disrupt vocal-fold vibration, they cause dysphonia and may also lead to airway obstruction. They are generally benign but rarely can have malignant transformation. The disease course can vary greatly and may stabilize in some patients, while progressing to severe obstructive disease requiring frequent excision in others. Treatment is surgical excision, with a limited role for topical and systemic therapy.2 Rosenberg et al10 showed a therapeutic advantage after human papillomavirus vaccination in patients with recurring respiratory papillomatosis, with vaccination resulting in fewer surgical procedures required and greater surgery-free intervals. Therefore, the AAO-HNS has published a position statement supporting vaccination for all patients ages nine to 45.11
Scar or sulcus vocalis is a furrow of the epithelium parallel to the vocal-fold edge, dampening the mucosal wave and decreasing pliability. Vocal-fold scars are similar but associated with deposition of fibrous tissue that deposits between the mucosal layer and the deeper muscular layer, causing stiffness of the vocal folds and resulting in dysphonia, vocal fatigue and increased vocal effort. These conditions may be congenital or caused by vocal trauma, misuse or surgery.12
Reinke edema is also referred to as polypoid corditis and involves swelling of the true vocal folds owing to accumulation of fluid within the potential space between the superficial lamina propria and the vocal ligaments (also called the Reinke space).2,13 The fluid accumulation is due to subepithelial hypervascularity, leading to vascular permeability and the disruption of collagen deposition. Reinke edema is almost exclusively caused by smoking, but vocal abuse or laryngopharyngeal reflux may also play a role.2 Primary treatment is smoking cessation, treating reflux and voice therapy. Severe obstructive Reinke edema may require surgery after these conservative treatments.13
MALIGNANT OR PREMALIGNANT VOCAL-FOLD LESIONS
Two-thirds of laryngeal cancer is located on the true vocal folds (glottic cancer).2 Squamous cell carcinoma accounts for over 90% of these cancers, and the earliest symptom is generally hoarseness.2 Because of this, 24% to 30% of glottic tumors are diagnosed in the early T1 stage, at which point the chances of local and distant metastases are extremely low, and five-year survival is nearly 100%.2 Treatment for early-stage glottic carcinoma is either transoral microsurgical resection or radiation therapy.2
If early symptoms are ignored and tumors present at later stages (T3 and T4), more radical resection or multimodality treatments are required, and five-year survival is much lower.14 Premalignant changes on the vocal folds may appear as keratotic lesions referred to as leukoplakia or as hypervascular, angiogenic-like lesions.15 Surgical pathology is necessary to provide definitive diagnoses.
Atrophy. Presbylarynx involves atrophy of the true vocal folds secondary to the natural physiologic aging process.2 As muscles and mucus-producing cells atrophy, the true vocal folds bow and prevent complete glottic closure. This leads to glottic insufficiency (where air escapes during attempted closure) and increases the surface viscosity of the mucus. These changes result in a hoarse, breathy, weak or strained voice.
Presbylarynx is present in up to 25% of patients over age 65.2 Initial treatment is voice therapy, but procedures to improve glottic competence are also an option.2 Bowing and atrophy are not exclusive to elderly patients and can also be seen in patients who have experienced significant weight loss or general debility.
Paresis or paralysis. A large spectrum of vocal-fold hypomobility exists. Complete immobility is generally referred to as paralysis whereas hypomobility is referred to as paresis. Resulting dysphonia is from incomplete glottic closure or irregular vocal-fold motion.2 The most common cause of vocal-fold paralysis is iatrogenic owing to surgery or trauma along the course of the recurrent laryngeal nerve. Surgeries that frequently risk the integrity of the nerve include thyroidectomy and parathyroid, carotid, cardiac, thoracic and cervical spine surgery.
A new vocal-fold paresis may also be a symptom of compression of the recurrent laryngeal nerve by a tumor such as thyroid or lung carcinoma. In 2% to 41% of cases, the cause of paresis cannot be identified and is termed idiopathic.2 Approximately 39% of unilateral idiopathic cases recover partial or complete motion, and 52% experience complete vocal recovery.16 If paresis persists past 12 months, recovery is unlikely.16 Initial treatment for paresis or paralysis is voice therapy or augmentation and medialization.2 Temporary measures (injection augmentation with filler materials) to procedurally medialize the true vocal folds are often employed to improve voice, airway protection, and cough strength. However, after 12 months of paresis, more permanent surgical procedures (such as type I thyroplasty or reinnervation) may be considered.2
Spasmodic dysphonia is a dystonia affecting the laryngeal muscles and has two different subtypes, adductor and abductor.2 Adductor spasmodic dysphonia is more common (90% of cases) and presents with a pressed, creaky voice caused by the vocal folds inappropriately pushing closed against each other. Conversely, abductor spasmodic dysphonia is rarer (10% of cases) and causes breathy voice breaks owing to the vocal folds inappropriately opening during phonation.2 Spasmodic dysphonia is treated with injection of botulinum toxin into the affected muscles to weaken them and improve stability of the voice.2
Vocal tremor is an involuntary rhythmic contraction of the laryngeal musculature resulting in dysphonia.17 Essential tremor is a generalized neurologic condition that may affect several structures (e.g., hands, limbs, head) or specific structures in isolation, such as musculature impacting the voice. Essential vocal tremor is the most common type of vocal tremor and is predominantly found in females (90.6%), with an average onset age of 70.17 It first presents as increased vocal effort but eventually progresses to obvious and disruptive fluctuations in the frequency and amplitude of phonation. Vocal tremor is difficult to treat, and there are currently no management guidelines. Some proposed treatment strategies include pharmacologic management, botulinum toxin injection, vocal-fold augmentation and deep brain stimulation.17
Other central and peripheral neurologic disorders. Parkinson disease is caused by degeneration of dopaminergic neurons of the substantia nigra, resulting in a movement disorder. Symptoms manifest as resting tremor, bradykinesia, rigidity and loss of coordination, with 70% to 89% of patients experiencing dysphonia with disease progression.18 Patients with Parkinson disease generally have difficulty initiating and controlling their rate of speech, and the voice is often described as tremulous, breathy, soft, monopitch or harsh. Laryngoscopy will generally show decreased sensation, bowing of the true vocal folds, glottic insufficiency and slowed vibration.
Parkinson disease is pharmacologically treated with levodopa. Unfortunately, there is no strong evidence that levodopa improves phonation, and other treatment strategies are generally employed. Voice therapy with a specific modality called the Lee Silverman Voice Treatment program is highly effective in Parkinson disease patients and is only performed by specially trained speech language pathologists. Other procedural interventions such as vocal-fold augmentation (“plumping” up the vocal folds by injecting temporary filler materials into them) can also be employed to help increase volume and strength of phonation.18
Other neurologic disorders that can result in dysphonia include myasthenia gravis and Eaton-Lambert disease. Therefore, when clinicians evaluate patients with dysphonia, it is vital to perform a thorough neurologic examination and keep a broad differential diagnosis in mind.18
Systemic diseases such as amyloidosis, rheumatoid arthritis, lupus, granulomatosis with polyangiitis (Wegner granulomatosis), sarcoidosis, tuberculosis, and several others may manifest with laryngeal pathology, leading to hoarseness. Treatment requires a multidisciplinary approach to treat the underlying disease process while also observing and managing laryngeal manifestations. Esophageal reflux can also cause backflow of refluxate into the larynx, resulting in irritation, laryngitis and hoarseness.2
WHO CREATED THEM AND HOW ARE THEY DETERMINED?
The original guidelines were put forth by the AAO-HNS in 200919 and updated in 2018.1 The Academy has a well-established system for creating guidelines that involves a systematic literature search with review by an expert committee. The information is then used to create key action statements with associated strengths of recommendation: strong recommendation, recommendation, option, recommendation against and strong recommendation against.20 The current guidelines have 13 key action statements.1
MAIN RECOMMENDATIONS
The AAO-HNS has published key action statements and recommends the following to clinicians to identify and manage dysphonia1:
CHANGES FROM PREVIOUS GUIDELINES
It is important to note that the 2018 guidelines1 are updated and vary from the previous 2009 guidelines.19 Changes include the following:
HOW WILL THE GUIDELINES IMPACT THE WAY CLINICIANS PRACTICE?
One purpose of these guidelines is to avoid delayed diagnosis of treatable benign lesions, systemic diseases, and early malignancies with an aim to impact the order in which clinicians evaluate and treat hoarseness. Further, pharmacologic management and imaging should not take place before direct visualization. This helps avoid unnecessary costs and complications associated with drug therapy and imaging. The guidelines also serve to appropriately funnel patients to speech therapy, which may not be a typical part of the clinician’s treatment algorithm.
While the terms hoarseness and dysphonia may be used interchangeably, hoarseness is a patient-reported symptom while dysphonia is diagnosed by a clinician as altered vocal quality. Hoarseness guidelines from the AAO-HNS assist clinicians in treatment and referral of patients. It is important to recognize that vocal fold pathology can range from benign to malignant, and that ignoring unresolved hoarseness or treating it with inappropriate pharmacotherapy can decrease quality of life or increase the risk of morbidity and mortality when associated with more serious conditions. Referral for direct visualization with laryngoscopy should be prompted by the noted red flags, four weeks of unresolved dysphonia or any suspicion for underlying disease.
REFERENCES
Advertisement
New innovations in upper airway care and insights into the Pediatric Voice Center
Insight on larynx disorders and treatments
A wide-ranging discussion on the latest in laryngology
A multidisciplinary approach and individualized plan of care are imperative
3 pediatric gastroenterologists discuss advances in their diagnostic toolkits
Injections and surgical procedures are among the therapies for patients with early and late vocal cord paralysis
A recently published case series highlights the broad range of laryngeal findings that can present among individuals with EDS
Analysis of HNSCC patients shows HPV status to be predictive of higher abundance of oncobacteria within the tumor