Three clinical trials testing old and new drugs
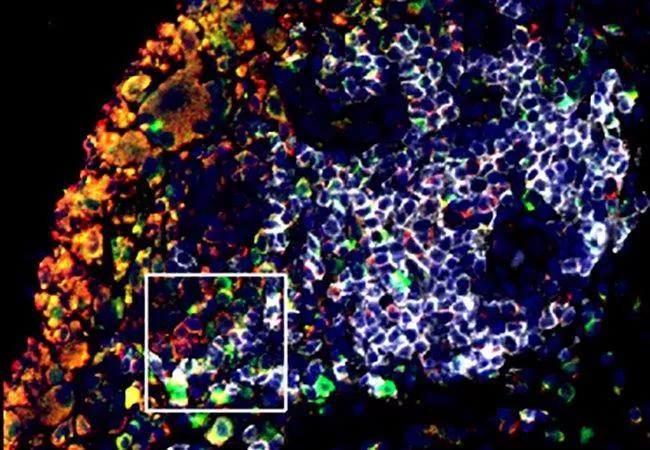
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5ed69f5b-abfc-4963-84bf-42423ebcc334/18-PUL-5877_jpg)
18-PUL-5877
Pulmonary arterial hypertension (PAH) and interstitial lung disease (ILD) number among the leading causes of morbidity and mortality in patients with rheumatic disease. Patients with concurrent rheumatic and lung disease can be a highly challenging group to treat.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Cleveland Clinic’s Rheumatic Lung Disease Program is currently engaged in three major multicenter trials whose common aim is to improve outcomes for these complex patients. The three trials detailed below are exploring the repurposing of old drugs and the creation of a new one to expand options for treating the devastating pulmonary complications of rheumatic disease.
Systemic scleroderma (Ssc), a devastating disease of unknown origin, causes fibrosis in multiple organs, leading to chronic morbidity and early death. The lungs often become involved, and such involvement portends a poor prognosis. Patients with SSc-ILD have a median survival of five to eight years.
Historically, clinicians have treated SSc-ILD patients with cyclophosphamide, which has a modest effect on forced vital capacity (FVC). However, that drug’s toxicity limits the amount of time in which it can be used.
Today, mycophenolate mofetil (MMF) is often used to treat SSC-ILD, based on the findings of the Scleroderma Lung Study II. Like cyclophosphamide, MMF has a modest effect on FVC, but it is less toxic.
The multinational SENSCIS© study is exploring yet another drug, nintedanib, as a safer alternative. Nintedanib is an antifibrotic agent that can slow the rate of decline in forced air volume, and is approved for treatment of idiopathic pulmonary fibrosis (IPF).
This randomized, placebo-controlled study compares nintedanib with placebo in 580 patients with SSC-ILD, and is the largest such trial to date. Patients may be on baseline MMF or methotrexate. Kristin Highland, MD, a Cleveland Clinic pulmonologist, and Dr. Oliver Distler of the University Hospital in Zurich, Switzerland, are co-leaders of the study.
Advertisement
In the SENCIS© study, the primary outcome measure is the annual rate of decline in forced air volume over 52 weeks. The two secondary measures are the patient’s skin fibrosis score and a quality-of-life measurement. For more detail about this study, see this post.
One to two percent of the general population develops rheumatoid arthritis (RA), a chronic inflammatory disease in which ILD is a common comorbidity. Today’s treatments for RA have proven highly beneficial in addressing the joint-related aspects of the illness. However, those treatments have not proven effective in addressing RA-associated lung disease, which can be lethal.
When ILD develops in a patient with RA, it multiplies the risk of death by nearly three times. Overall, ILD is responsible for about 10 percent of deaths in RA.
The TRAIL-1 study aims to address this gap in treatment. A multicenter, randomized, placebo-controlled study, it is testing the safety and tolerability of pirfenidone versus placebo for the treatment of RA-ILD.
Perfenidone, an antifibrotic agent, currently has FDA approval for the treatment of IPF, based on its ability to slow the rate of decline in FVC. The drug is thought to have both anti-inflammatory and antifibrotic mechanisms, making it an ideal candidate for investigation in RA-ILD. Read more about this study here.
PAH is often associated with connective tissue disease (CTD) as well as other medical conditions. However, the response of patients with systemic sclerosis to PAH-specific therapy appears to be worse than in other forms of PAH. Approved vasodilation treatments do not yield significant functional improvements in CTD-PAH patients.
Advertisement
Meanwhile, bardoxolone methyl and its analogs have potent effects to counter inflammation, mitochondrial dysfunction and autoimmune processes that are directly applicable to treatment for WHO Group 1 CTD-PAH patients.
The Catalyst study is a phase 3, multinational, placebo-controlled study of bardoxolone versus placebo for CTD-PAH. The primary endpoint is change from baseline in six-minute walk distance. The secondary endpoint is time to first clinical improvement, as defined in several ways. Learn more about this study here.
With unique expertise in the intersection of rheumatic and lung diseases and involvement in multiple, national clinical trials, Cleveland Clinic’s Rheumatic Lung Disease Program serves as a resource for clinicians and patients. To refer a patient or learn more, please contact me or call 855.REFER.123.
Dr. Highland is board certified in rheumatology, pulmonary medicine and critical care and directs the Rheumatic Lung Disease Program in Cleveland Clinic’s Respiratory Institute
Feature image: B Cells Contribute to Bone Loss in Rheumatoid Arthritis
Joint tissue from patients with rheumatoid arthritis (RA) contains high numbers of B cells (white), the antibody-producing cells of the immune system. These cells produce high levels of the signaling molecule RANKL (green), which stimulate osteoclasts (red), causing bone to break down. Researchers aim to identify the specific B cell subsets and molecular pathways involved in the cells’ harmful effects so that they can find ways to target them selectively. The ultimate goal is to develop new RA treatments with fewer unwanted side effects. Credit: Jennifer Anolik, MD, PhD, University of Rochester. Republished from NIH Image Gallery under creative commons license.
Advertisement
Advertisement
Takeaways from the most recent annual meeting centered around clinical advances, AI integration and professional development
Recent breakthroughs have brought attention to a previously overlooked condition
A review of treatment options for patients who may not qualify for surgery
Looking at the real-world impact and the future pipeline of targeted therapies
The progressive training program aims to help clinicians improve patient care
New breakthroughs are shaping the future of COPD management and offering hope for challenging cases
Exploring the impact of chronic cough from daily life to innovative medical solutions
How Cleveland Clinic transformed a single ultrasound machine into a cutting-edge, hospital-wide POCUS program