Evidence mounts that inflammation plays a key role
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Subarachnoid hemorrhage from a ruptured intracranial aneurysm, while uncommon, has devastating consequences: It leads to death in about 50% of cases, while about half of those who survive are left with substantial disability. Understanding why intracranial aneurysms occur in 3% to 5% of the population — and why nearly 0.5% of those rupture annually — is critical to developing strategies for prevention.
Multiple interacting processes have been implicated in aneurysm formation and rupture. Long-established risk factors — including anatomy, genetics, connective tissue disorders, hypertension and smoking — help determine vulnerability. Recently, increased focus has been placed on the role of inflammation in causing vascular smooth muscle dysfunction, driving both aneurysm formation and rupture, especially in vulnerable individuals.
Aneurysms typically develop at points of stress in the circulation — i.e., at bifurcations and vascular angles. The anterior and posterior communicating arteries are particularly susceptible, as is the posterior circulation in general, especially the vertebral and basilar arteries (Figure 1).
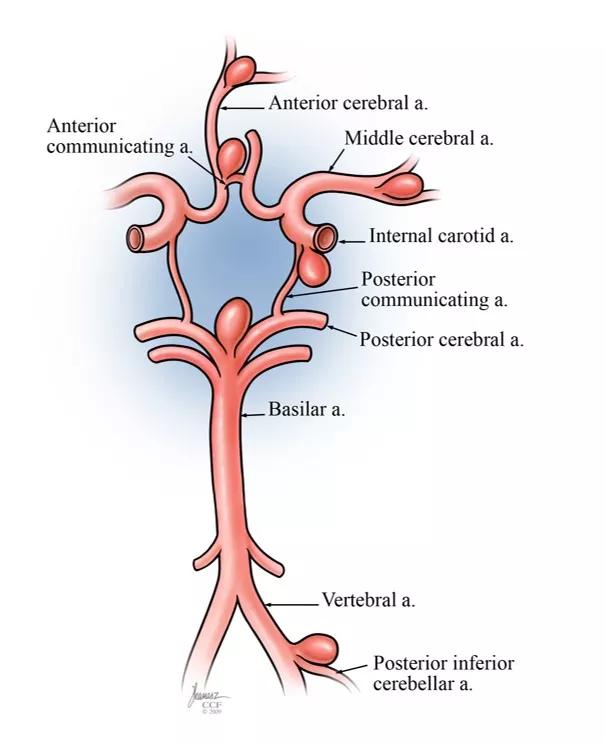
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/29672e0a-a953-431e-b25a-49f9b189a01c/19-NEU-6069_Inset1-intracranial-aneurysm_jpg)
Figure 1. Illustration showing common sites for intracranial aneurysm development.
Histologically — starting from the interior lumen — the layers of a cerebral blood vessel are as follows:
Advertisement
The propensity of cerebral arteries to develop aneurysms stems from their thinner adventitia relative to systemic arteries and the reduced proportion of smooth muscle cells and elastin fibers in the media (Figure 2).
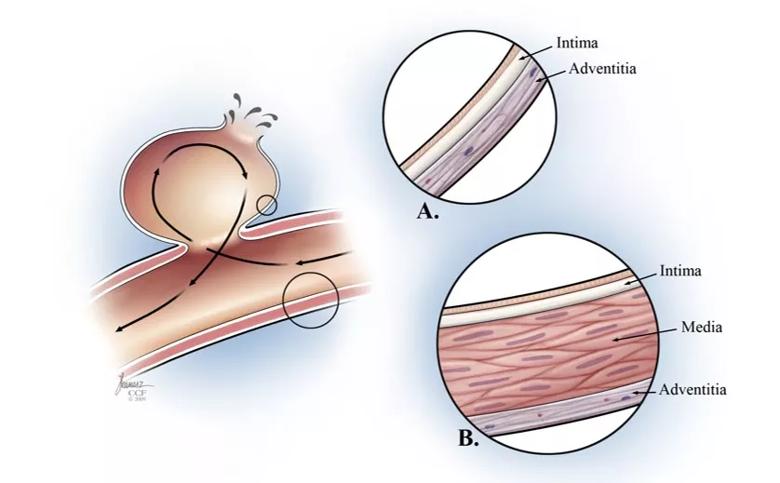
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/af518a32-97de-48b0-80d7-4898d306401c/19-NEU-6069_Inset2-cerebral-blood-vessel-layers-in-intracranial-aneurysm_jpg)
Figure 2. Illustrations showing layers of a cerebral blood vessel (insets A and B) in the context of an intracranial aneurysm (left panel).
Aneurysm size is the traditional method of determining rupture risk, with aneurysms smaller than about 4 mm in diameter generally considered safe for periodic monitoring, depending on location and patient risk factors.
Nevertheless, small aneurysms are actually a common cause of subarachnoid hemorrhage, and recent evidence has identified two measures that confer greater predictive power for rupture of small aneurysms:
For both measures, a larger ratio is associated with higher risk. Other features conferring elevated rupture risk are an irregular shape or a “daughter sac,” i.e., a secondary bulge on the aneurysm.
Such morphologic features affect flow dynamics on the endothelium and vessel wall. Paradoxically, lower hemodynamic stress can confer higher risk, by triggering apoptosis of endothelial cells and vessel wall degeneration, leading to aneurysm growth and increased risk of rupture.
Aneurysms tend to run in families. If one relative has had a ruptured aneurysm, the risk doubles; if two relatives, the risk increases fourfold.
Advertisement
Specific gene loci — including 7q11, 14q22, 5q22-31, 19q13.3 — have been tied to aneurysms that are related to coding of elastin and collagen. For now, genetic testing in patients with cerebral aneurysms is confined to the research arena, where it offers an intriguing focus for understanding underlying physiological mechanisms and developing therapeutic strategies.
In addition, conditions that predispose to intracranial aneurysms include:
Smoking affects essentially every step of aneurysm formation and rupture and is the most significant modifiable risk factor. Effects of smoking on cerebral arteries include hemodynamic stress and formation of reactive oxidative species that contribute to inflammatory processes and endothelial dysfunction.
Hypertension is another major risk factor. High blood pressure alters flow dynamics, causing abnormal wall shear stress and leading to cerebral vessel endothelium disruption.
Trauma, infection and female sex are also linked to increased risk.
The role of inflammation in aneurysm pathogenesis is an important focus of research. Current evidence points to a complex interplay between endothelial dysfunction, inflammatory responses and flow dynamics that eventually leads to aneurysm rupture.
The following picture is emerging: Areas of vessel dysfunction or injury attract macrophages, mast cells and T cells, which then contribute to alteration of vascular smooth muscle cells. As macrophages release cytokines and other mediators, matrix metalloproteinases cleave the extracellular matrix, inducing a positive feedback cycle that further promotes migration of inflammatory cells.
Advertisement
While normal vascular smooth muscle cells provide collagen synthesis and maintenance of a healthy extracellular matrix, inflammation causes disruptive remodeling, resulting in thinning of the internal elastic lamina and media, and eventual apoptosis of the vascular smooth muscle cells. Such injury induces further inflammation. Eventually the integrity of the vascular wall is compromised, resulting in aneurysm development and rupture.
1) Patients with a known or newly discovered intracranial aneurysm should be evaluated and monitored by a neurospecialist with expertise in cerebral aneurysms. Assessing the risk of rupture must be weighed against the risks of intervention.
2) While a patient is being monitored, the two most important steps in clinical care are to bring hypertension under control and help the patient stop smoking.
3) Aspirin therapy is associated with a reduced risk of rupture but also an elevated risk of bleeding. The jury is still out on whether it should be routinely advised.
4) Statin therapy has been proposed, but clinical studies have not shown benefit.
5) Stay tuned for anti-inflammatory strategies, which remain an important area of active study.
Dr. Hussain, Director of Cleveland Clinic’s Cerebrovascular Center, specializes in vascular neurology and endovascular surgical neuroradiology. This article is derived from a presentation he gave at the American Heart Association’s 2019 Scientific Sessions.
Advertisement
Advertisement
Guidance from the largest cohort of SEEG-confirmed insular epilepsy patients reported to date
Ethical guidance provides guardrails so medical advances benefit patients
OCEANIC-STROKE results represent long-sought advance in secondary stroke prevention
Two studies from Cleveland Clinic may help advance the technology toward broader clinical use
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
An argument for clarifying the nomenclature
An expert talks through the benefits, limits and unresolved questions of an evolving technology
Recommendations on identifying and managing neurodevelopmental and related challenges