Leveraging physiologic forces to achieve hemostasis
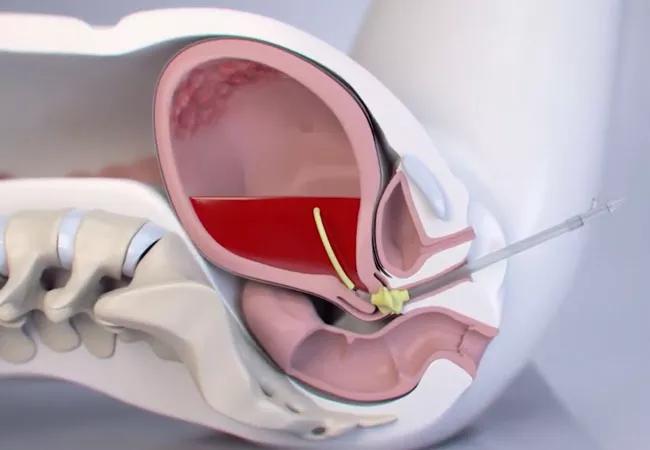
In most deliveries, after the placenta has emerged, the muscle fibers in the myometrium constrict, causing the uterus to contract and bleeding to stop. But for patients with atony, the process fails and postpartum hemorrhage — a leading cause of maternal deaths from obstetric causes — ensues.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Now a new intrauterine vacuum device offers hope of preventing severe maternal morbidity and mortality by quickly and safely treating abnormal postpartum uterine bleeding or postpartum hemorrhage. Called the Jada® System, it was recently approved by the U.S. Food and Drug Administration based on results of a prospective, single-arm study at 12 medical centers in the United States.
“The success rate with the device in the clinical trial was 94% and it was effective in a median of 3 minutes,” says Edward Chien, MD, MBA, Chair of the Department of Obstetrics and Gynecology within Cleveland Clinic’s Ob/Gyn and Women’s Health Institute. “Only 5% of patients required use of a second or third intervention for continued bleeding, which is impressive compared with results of other uterotonic agents.”
The trial enrolled 107 women aged 18 years or older with normal uterine anatomy and placentation who delivered at ≥ 34 weeks’ gestation. Before use of the vacuum device, all had atony-related blood loss of 500–1500 mL or 1000–1500 mL following vaginal or cesarean delivery, respectively. Blood loss criteria were developed acknowledging the American College of Obstetricians and Gynecologists’ revitalize definition.
The mean maternal age in the study was 29.7 years; 57% of the women were white and 24% were Black. Nearly two-thirds (64%) of the participants were obese. Eighty-five percent of the deliveries were vaginal.
The primary effectiveness endpoint was successful treatment of uterine atony, defined as no need for other open surgical or nonsurgical interventions after application of the Jada System. The primary safety endpoint was incidence and severity of device-related adverse events (AEs), with data collected from enrollment to the 6-week follow-up visit.
Advertisement
“Failure to control bleeding occurred in only one case and infection was reported in only 7% of cases,” says Dr. Chien, who co-authored the report on the study that appeared in Obstetrics & Gynecology. “That issue was probably unrelated to the device itself because women with postpartum bleeding have higher rates of infection.”
The eight device- or procedure-related AEs in the study — none of which required treatment — were endometritis (n = 4), disruption of a vaginal laceration repair (n = 1), presumed endometritis (n = 1), bacterial vaginosis (n = 1) and vaginal candidiasis (n=1). There were no cases of uterine rupture, lower genital tract laceration or uterine incision dehiscence related to use of the Jada System.
After use of the device, 35 participants required transfusion of 1 to 3 units of red blood cells (RBCs) and five participants required 4 or more units of RBCs.
Made of medical-grade silicon, the Jada System consists of an elliptical intrauterine loop on the distal end and a vacuum connector on the proximal end. Standard tubing is used to connect it with an inline graduated canister and regulated vacuum source. Twenty vacuum pores on the inner surface of the loop facilitate creation of a vacuum in the uterine cavity. On the outside, the pores are covered by a shield, protecting maternal tissue.
The device is introduced through the cervix into the uterine cavity, with the doughnut-shaped cervical seal just outside the external cervical os. The cervical seal is filled with 60–120 mL of sterile fluid and a low-level vacuum (80±10 mm Hg) is applied and the device left in place with the vacuum on for at least 1 hour. Once bleeding is controlled, the vacuum is disconnected, the cervical seal emptied, and the device left for at least another 30 minutes.
Advertisement
Says Dr. Chien, “The suction from the device causes the uterus to collapse. The muscles fibers then obstruct the arteries that are perforating through the muscle to the lining of the uterus. That mimics the way postpartum blood flow is regulated and it’s actually more physiologic than other intrauterine systems that inflate and potentially allow blood vessels to remain open.”
Some 98% of investigators in the trial said that the device was easy to use and 97% would recommend it. Based on the positive data from the study and with the FDA approval, the Jada System is now being rolled out for use in Cleveland Clinic’s three major maternity centers. Dr. Chien believes it can not only reduce maternal morbidity and mortality but also reduce costs associated with labor and delivery.
“This device is easy to use and the response rate was impressive. Patients who responded were able to be transferred to normal postpartum units quickly instead of staying in labor and delivery for 24 hours,” he says. “Our focus in maternal-fetal medicine is on patient safety and reducing pregnancy-associated morbidity and mortality, and this device is should help to improve outcomes.”
Advertisement
Advertisement

Uterine transposition cleared the field for radiation therapy

ACOG-informed guidance considers mothers and babies

Prolapse surgery need not automatically mean hysterectomy

Artesunate ointment shows promise as a non-surgical alternative

New guidelines update recommendations

Two blood tests improve risk in assessment after ovarian ultrasound

Recent research underscores association between BV and sexual activity

Psychological care can be a crucial component of medical treatment