First-ever U.S. population-level retrospective analysis reveals many patients with systemic mastocytosis need faster intervention
Until recently, common wisdom held that patients with indolent systemic mastocytosis (ISM) had a slow-moving course of disease. However, a new study revealed that many patients in this category experience rapid progression and lower survival than previously thought.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The first-ever population-level study of systemic mastocytosis (SM) patients in the U.S. found that ISM had a modest but statistically significant decrease in survival, even early in the disease course (in the first four years). This occurred even among those with no evidence of progression to advanced disease.
Affecting roughly 50,000 people across the U.S., SM is a rare blood disorder that increases the number of abnormal mast cells in bone marrow and sometimes other organs or sites throughout the body. This condition is characterized by a high propensity for allergic manifestations to a wide variety of triggers and a strong underlying inflammatory component, which has long-term consequences affecting virtually any organ. It's not unusual for these patients to have high symptom burden and to develop dysfunctions to multiple organs such as gastrointestinal, kidney, liver, spleen, lungs and skin.
An estimated 15% of patients with the disease have what’s characterized by the World Health Organization as advanced systemic mastocytosis while the majority of the remaining patients have ISM. Although patients with ISM usually have bone marrow involvement, they typically do not have other organ involvement. The indolent version of the disease has long thought to have a less severe clinical course.
In a large, recently completed population study, the findings of which were presented at the American Society of Hematology annual meeting in December of 2022, Cleveland Clinic researchers and other investigators found that patients with ISM are very heterogeneous with regards to symptoms and need for supportive care. Building on that study, they then conducted a population-level study of ISM in the U.S. using a health claims-based dataset to analyze overall survival.
Advertisement
From a health claims database of 320 million patients across the U.S. between 2015 and 2022, the researchers identified 8,332 patients with ISM using a novel claims-based algorithm. For comparison, they established an independent control cohort of 2,000 patients without SM matched by age, sex, race/ethnicity, comorbidities and insurance status.
The researchers found a statistically significant decrease in overall survival between the ISM group and the non-SM control cohort. Higher mortality was found in patients with ISM, regardless of whether there was evidence of disease progression.
“The perception had been that it took a long time for indolent systemic mastocytosis to advance to aggressive disease, but our data from the U.S. is showing that is not the case. And in some instances, patients are progressing in a much shorter time than previously thought,” says Sudipto Mukherjee, MD, PhD, MPH, Director of Rare Cancers and Blood Diseases at Cleveland Clinic Cancer Institute.
Not all patients with an indolent diagnosis will behave biologically or clinically the same way. There is a high degree of variability in patients with ISM in terms of symptom burden. Some are asymptomatic while others have a high symptom burden, requiring a large number of medications to control symptoms. Those with a high symptom burden are more likely to progress to advanced systemic mastocytosis.
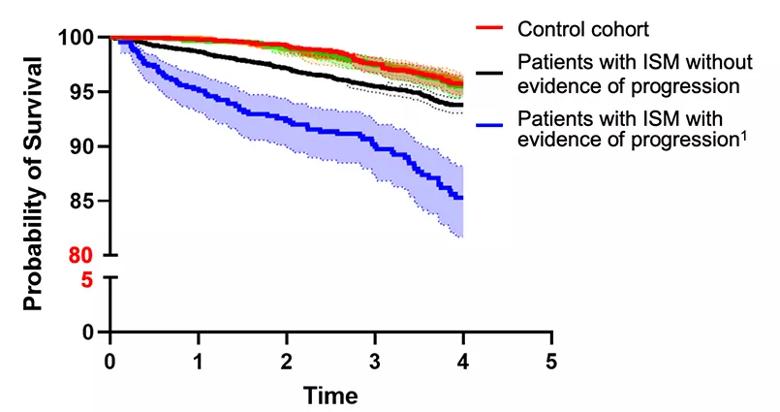
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2a0b1e9f-cf75-451c-90b8-47b20982a5c3/survival-indolent-systemic-mastocytosis)
“There is an under appreciation of the fact that patients with ISM may not have as long a survival as previously thought,” cautions Dr. Mukherjee. “This data provides additional evidence for treating providers to consider early initiation of KIT inhibitors in ISM patients.” Further research is needed to find out the biological reasons why some ISM patients progress more quickly to advanced SM.
Advertisement
The hope is that there is room for improving the survival of patients with ISM through appropriate identification and earlier treatment. These findings should give physicians and patients more nuance in shared decision-making about the timing of initiating treatment.
In June 2021, the FDA approved the KIT inhibitor avapritinib for patients with indolent disease. As a disease-modifying therapy, it is thought the medication not only controls symptoms but will delay disease progression. "A bigger question is, do all ISM patients need to be treated immediately, regardless of symptom burden?” says Dr. Mukherjee. “As with any drug, there is a risk-benefit analysis involved.” More data is needed to make this determination.
In the short term, researchers are seeking to understand if this medication can help to stave off organ dysfunction or other disease complications. As more data like this comes to light, this may provide insights into the way patients are categorized and cared for.
Advertisement
Advertisement
Education, prevention strategies and monitoring serves this at-risk group

As the pandemic evolves, rheumatologists must continue to be mindful of most vulnerable patients

Early results suggest positive outcomes from COVID-19 PrEP treatment

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer