Safety is focus of pilot study in a high-risk population
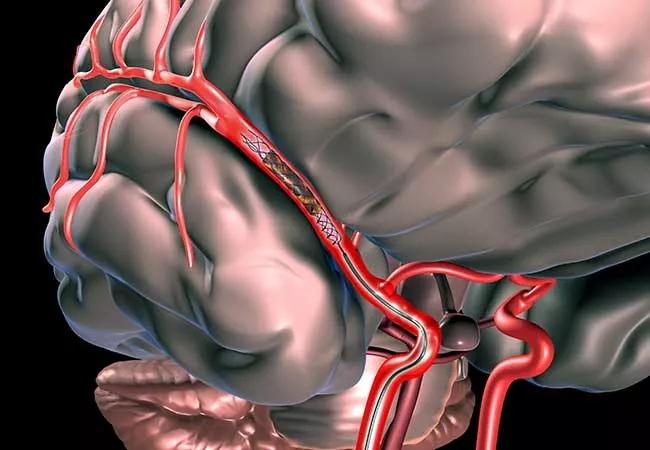
Are patients with minor stroke symptoms and intracranial large vessel occlusions appropriate candidates for mechanical thrombectomy? This largely neglected question is now getting attention from an ongoing pilot study at Cleveland Clinic.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Patients presenting with mild stroke symptoms often receive no treatment, but it’s increasingly recognized that those with large vessel occlusions may represent a subgroup at risk for poor outcomes.
“In the past these patients typically didn’t undergo vessel imaging, so we often didn’t know that they had occlusions,” says Gabor Toth, MD, a vascular and interventional neurologist with Cleveland Clinic’s Cerebrovascular Center.
“That is changing,” he adds. “As we image more patients early on to look for vascular problems, we’re finding more and more patients with mild strokes and large occlusions. And the retrospective data tell us these patients don’t do as well.”
Mechanical thrombectomy has been shown in several randomized controlled trials to improve outcomes in patients with acute ischemic stroke, and American Heart Association/American Stroke Association guidelines now recommend mechanical thrombectomy to remove vessel occlusions and/or tissue plasminogen activator (tPA) when appropriate.
But the guidelines do not address patients with mild stroke symptoms, and this population has not been represented in studies of stroke therapies, Dr. Toth explains. That lack of data is especially vexing since mechanical thrombectomy carries risks and expenses inherent to any invasive procedure, he adds.
This is the backdrop for the recent launch of the prospective MISTWAVE (Mild acute Ischemic STroke With lArge VEssel occlusion) registry, whose main goal is to assess the safety of mechanical thrombectomy in the removal of large vessel occlusions in patients with mild stroke symptoms.
Advertisement
Dr. Toth and Cleveland Clinic colleagues are currently enrolling patients in the single-arm pilot study, with the goal of 20 enrollees. Eligible patients have mild stroke, defined as a National Institutes of Health Stroke Scale (NIHSS) score < 6, and receive best medical therapy combined with endovascular mechanical thrombectomy using one of the current FDA-approved devices. Patients can be enrolled regardless of whether or not they’ve received tPA.
Primary end points are:
Secondary end points include several efficacy measures — i.e., successful angiographic recanalization, 30-day global disability assessment and infarct volume measures on CT or MRI
Dr. Toth will present preliminary findings from the first three study enrollees in a poster presentation at the annual meeting of the Society of NeuroInterventional Surgery in late July.
He says the results so far are very encouraging. “We’ve been able to open vessels and restore blood flow in all three patients with no complications,” he notes. “These patients have all been discharged following the procedure without need to go to rehabilitation. At follow-up within a few weeks of discharge, they were basically back to their neurologic baseline.”
The pilot study may soon expand to two other research centers, and Dr. Toth says he hopes to recruit 15 to 20 patients within the next six months.
Advertisement
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Rapid innovation is shaping the deep brain stimulation landscape

Study shows short-term behavioral training can yield objective and subjective gains

How we’re efficiently educating patients and care partners about treatment goals, logistics, risks and benefits

An expert’s take on evolving challenges, treatments and responsibilities through early adulthood

Comorbidities and medical complexity underlie far more deaths than SUDEP does

Novel Cleveland Clinic project is fueled by a $1 million NIH grant

Tool helps patients understand when to ask for help